New fungicides that provide a new level of efficacy, along with integrated management practices can help California citrus growers deliver high-quality fruit to valuable export markets.
In a Citrus Research Board webinar, UC Riverside plant pathologist Jim Adaskaveg cited pre- and post-harvest diseases that infect citrus fruit when environmental conditions including rainfall favor spread of the fungal pathogens.
“All pre harvest diseases need high rainfall to develop and cause economic damage,” Adaskaveg said. Most post-harvest diseases have their origins in fruit injuries that allow entry of pathogens, he added.
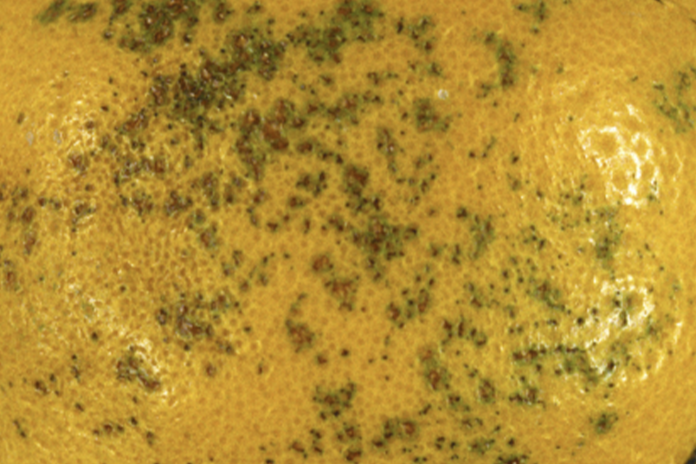
Two citrus fungal diseases that will keep citrus fruit out of the export market are Septoria spot and Phytopthora. Infections of Septoria spot begin with injury to the peel. An ice mark due to a freeze event will leave small irregular pitted, shallow lesion on fruit and an entry point for a pathogen. As the infection advances, the lesions become dark and extend into the albedo- the white part of the peel. The disease does not affect the juice quality, but the appearance of the fruit will limit marketing opportunities. S citri, the pathogen that causes Septoria spot, is a quarantine pest in South Korea, a major market for California citrus. Adaskaveg said the Korean market for citrus is maintained through compliance with quarantine measures, following GAPs and fruit certification in the NAVEK program (a joint UC/citrus industry program).
Phytopthora, is a soilborne disease that can affect tree roots, trunk and the fruit. This complex disease can cause tree decline, root rot, brown rot and yield reduction A pre-harvest infection of brown rot is most likely to be found on fruit growing on the lower one-third of the tree as water splashing from rain or irrigation can spread the pathogen up into the tree.
Orientation of the orchard, tree density and skirting are cultural practices that can help keep infections low pre-harvest. Complementary strategies include pre-harvest fungicide treatments and implementing handling procedures to minimize injury. Planting tolerant rootstocks in areas where Phytopthora infections are common is recommended.
Adaskaveg said new fungicides registered for use in citrus are highly effective in reducing Phytopthora soil populations and root rot as well as improving yields. Two of the new formulations are Orondis and Presidio.
These new fungicide each have a different mode of action and field studies have shown that the active ingredient in these formulations were detected in root, stem and leaf tissues of treated citrus seedlings indicating systemic uptake.
The new fungicides are expected to reduce dependence on fumigation of orchard sites and provide resistance management for sustainable control of Phytopthora.