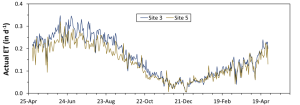
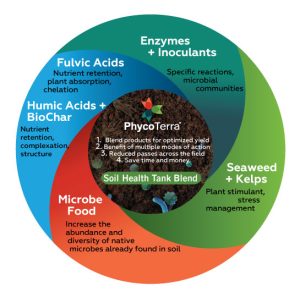
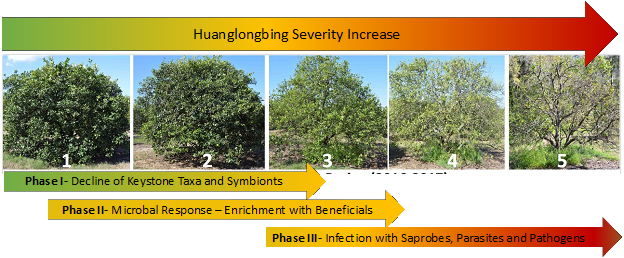
Berry sugar and anthocyanin accumulation are key factors in determining the fruit quality of red wine grapes in the San Joaquin Valley (SJV), where >70% of California wine grapes are grown (California Grape Crush Report 2022). Hot climates are not ideal for red Bordeaux cultivars such as Cabernet Sauvignon and Merlot as anthocyanin accumulation is inhibited (Figure 1).

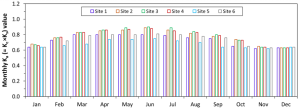
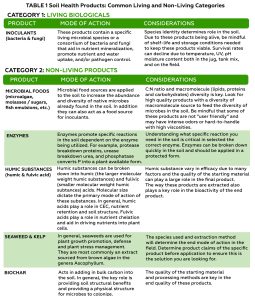
However, fruit quality might be improved with certain management practices, including deficit irrigation and leafing. Previous research in the SJV demonstrated that moderate irrigation deficits can improve grape yield and quality in addition to saving water (Williams 2012). Mild or moderate irrigation deficits promote yield formation due to increased bud fruitfulness and decreased fungal disease pressure. Sustained deficit irrigation (SDI) of 70% to 80% evapotranspiration (ETc) was found to balance economically sustainable yield, fruit quality and water-savings goals (Williams 2010). Abundant winter precipitation and overirrigation cause grapevines to grow excessively, shading the fruit, directly reducing quality and favoring the development of fungal diseases (Mendez-Costabel et al. 2014) (Figure 2).


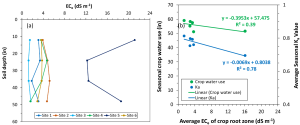
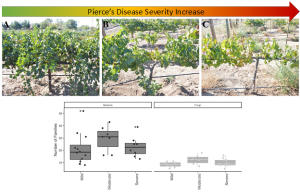
Years like 2023 might remind growers that managing water and canopy size to improve canopy microenvironment and enhance spray coverage will reduce fungal disease pressure (Figure 3). However, severe water deficits pre-veraison significantly impair grapevine vegetative and reproductive growth, photosynthesis and fruit maturity (Levin et al. 2020).

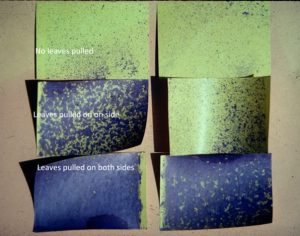
Removing leaves in the fruit zone is another beneficial practice growers may do to improve fruit quality. Leafing increases fruit exposure which may directly improve fruit quality, create a microenvironment that discourages powdery mildew and bunch rots, and improve spray coverage (Austin and Wilcox 2011) (Figure 4). Leaf removal is most practiced in cool climates as overexposure can easily reduce fruit quality in a hot climate. However, studies on leaf removal in a hot climate also showed similar benefits as reported in cooler climates (Cook et al. 2015). As with deficit irrigation, the timing and intensity of fruit zone leaf removal determines the potential impact on grapevine yield and fruit quality at harvest. In a cool climate, basal leaf removal prior to bloom may reduce berry set, thus lowering yield (Acimovic et al. 2016). Effects on berry set depend on the extent of leaf removal and the weather (Frioni et al. 2017). In hot climates, mechanical fruit zone leaf removal prior to bloom had no effect on berry set or yield (Cook et al. 2015). In addition to the potential to reduce set in cool climates, leaf removal prior to bloom can increase berry total soluble solids, anthocyanin content and berry aroma compounds (Ryona et al. 2008). Recently, mechanical fruit zone leaf removal has gained popularity due to labor shortage and increased labor cost in California (Kurtural and Fidelibus 2021). Years like 2023 which came with abundant winter precipitation, delayed harvest and cool temperatures might require additional fruit-zone leaf removal to open the canopy and increase spray coverage to help control fungal diseases.


Three-Year Field Study
Aiming to find the “sweet spot” of water management and leaf removal on yield, sugar and anthocyanin accumulation of red wine grape in hot climates, we conducted a three-year field study on Cabernet Sauvignon grown in Madera as Cabernet Sauvignon is believed to be one of the most challenging varieties to be grown in the SJV due to lack of berry color at harvest.
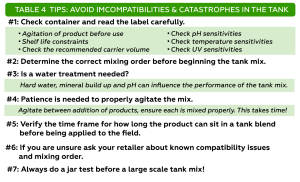
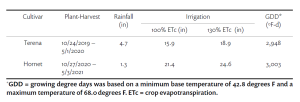
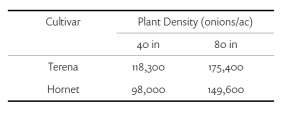
The experiment was conducted in a commercial vineyard located in Madera on fine sandy loam soil. 10-year-old Cabernet Sauvignon vines on Freedom rootstock with 4’ ´ 10’ spacing and Northeast-Southwest row orientation were used for the experiment. The grapevines were quadrilateral cordon trained with a 24-inch cross-arm to 48-inch height above vineyard floor with a pair of catch wires above the cordons. A two (deficit irrigation) × three (leaf removal) factorial split-plot design was applied for three seasons: 2018 through 2020. Two irrigation treatments were applied: 1) sustained deficit irrigation (SDI): water was maintained at 80% of weekly crop evapotranspiration (ETc) through the growing season; 2) regulated deficit irrigation (RDI): water was maintained at 50% ETc from berry set to veraison then switched back to 80% ETc until harvest. ETc was calculated using the equation of ETc = ETo × Kc (Williams 2010). On top of irrigation treatments, we applied three timings of mechanical leaf removal: 1) bloom, 2) berry set and 3) no leaf removal. Leaf removal was applied to both sides of the canopy using a roll-over leaf plucker with a sickle-bar sprawl clipper adapted for a sprawling-type canopy (Model EL-50, Clemens Vineyard Equipment, Woodland, Calif.).
Results and Discussion
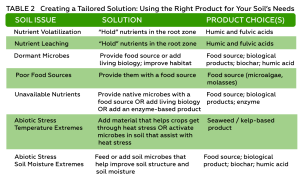
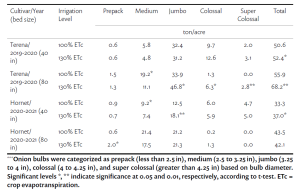
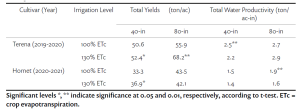
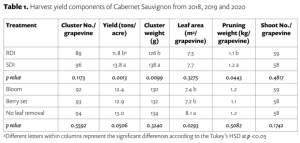
RDI reduced yield by 15% compared to SDI mainly due to smaller berries and clusters (Tables 1 and 2). Leaf removal did not significantly affect yield. Our result confirms that severe water deficit, like 50% ETc, pre-veraison, can result in significant yield loss. Contradictory to the previous field observation, bloom leaf removal had no effect on yield, and growers should be less worried about yield loss due to bloom leaf removal than severe deficit irrigation.
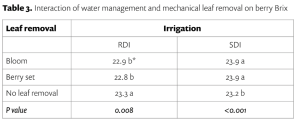
Berry soluble solids (Brix) were affected mainly by irrigation treatments in our study. RDI consistently reduced soluble solids each year (Table 2). Interestingly, we found that the effect on Brix depended on the interaction of leaf removal and water management (Table 3). Leaf removal increased Brix when vines were not water stressed or mildly stressed like when SDI was applied whereas leaf removal reduced Brix when vines were severely water stressed like when RDI was imposed. This implies to growers that if sugar is your biggest concern, you should water vines maintaining mild or moderate vine water stress and remove fruit-zone leaves.
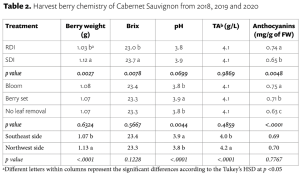
Berry anthocyanin content is critically important for red wine grapes. RDI increased berry anthocyanins by 14% in comparison of SDI, and bloom and berry set leaf removal increased anthocyanins by 19% and 13%, respectively, compared to no leaf removal control (Table 2). This means the 14% increase in anthocyanin concentration from the RDI treatment is proportional to the decrease in berry weight and yield. So, there is no net gain of anthocyanins per berry associated with the RDI irrigation treatment. Bloom leaf removal increased anthocyanins by nearly 20% with no yield reduction and that means bloom leaf removal provides a net gain of anthocyanins per berry.
Bloom leaf removal was more effective than pre-veraison RDI at improving berry Brix and anthocyanins without adversely affecting yield. Given the significant reduction on yield from severe deficit irrigation and the low economic return per ton of fruit in the SJV, bloom mechanical leaf removal coupled with SDI of 80% ETc could be a useful practice for SJV growers.
References
Acimovic, D., Tozzini, L., Green, A., Sivilotti, P., and Sabbatini, P. (2016) Identification of a defoliation severity threshold for changing fruitset, bunch morphology and fruit composition in Pinot Noir. Australian Journal of Grape and Wine Research, 22: 399– 408. doi: 10.1111/ajgw.12235.
Austin, C and Wilcox, W. (2011) Effects of Fruit-Zone Leaf Removal, Training Systems, and Irrigation on the Development of Grapevine Powdery Mildew. Am J Enol Vitic. June 2011 62: 193-198.
Cook, M., Zhang, Y., Nelson, C., Gambetta, G., Kennedy, J., Kurtural, K. (2015) Anthocyanin Composition of Merlot is Ameliorated by Light Microclimate and Irrigation in Central California. Am J Enol Vitic. 66: 266-278.
California Sustainable Groundwater Management Act (SGMA) 2014 Sustainable Groundwater Management Act (SGMA) (ca.gov)
California Grape Crush Report 2022, USDA National Agricultural Statistics Service (NASS). USDA – National Agricultural Statistics Service – California – Grape Crush Reports
Frioni, T., Zhuang, S., Palliotti, A., Sivilotti, P., Falchi, R. and Sabbatini, P. (2017) Leaf Removal and Cluster Thinning Efficiencies Are Highly Modulated by Environmental Conditions in Cool Climate Viticulture. Am J Enol Vitic. 68: 325-335.
Kurtural, K and Fidelibus, M. (2021) Mechanization of Pruning, Canopy Management, and Harvest in Winegrape Vineyards. Catalyst: Discovery in Practice. 5: 29-44.
Levin, A., Matthews, M., and Williams, L. (2020) Effect of Preveraison Water Deficits on the Yield Components of 15 Winegrape Cultivars. Am J Enol Vitic. 71: 208-221.
Mendez-costabel, M., Wilkinson, K., Bastian, S., Jordans, C., Mccarthy, M., Ford, C., and Dokoozlian, N. (2014) Effect of increased irrigation and additional nitrogen fertilisation on the concentration of green aroma compounds in Vitis vinifera L. Merlot fruit and wine. Australian Journal of Grape and Wine Research. 20:80–90.
Ryona, I., Pan, B., Intrigliolo, D., Lakso, A., and Sacks G. (2008) Effects of Cluster Light Exposure on 3-Isobutyl-2-methoxypyrazine Accumulation and Degradation Patterns in Red Wine Grapes (Vitis vinifera L. Cv. Cabernet Franc). Journal of Agricultural and Food Chemistry 56 (22), 10838-10846.
Williams, L. (2010) Interaction of rootstock and applied water amounts at various fractions of estimated evapotranspiration (ETc) on productivity of Cabernet Sauvignon. Australian Journal of Grape and Wine Research. 16:434–444.04
Williams, L. (2012) Interaction of applied water amounts and leaf removal in the fruiting zone on grapevine water relations and productivity of Merlot. Irrig Sci. 30: 363-375.
Williams, L. (2014) Effect of Applied Water Amounts at Various Fractions of Evapotranspiration on Productivity and Water Footprint of Chardonnay Grapegrapevines. Am J Enol Vitic. 65: 215-221.