Carrots are one of the 10 major commodities in Imperial County, with an average acreage of nearly 16,000 over the past decade. The farm gate value of fresh market and processing carrots was about $66 million in 2019. In the low desert region, fresh market and processing carrots are planted from September to December for harvest from January to May. Most carrots are typically sprinkler irrigated for stand establishment and subsequently furrow irrigated for the remainder of the growing season. However, there are fields that are irrigated by solid set sprinkler systems the entire crop season.
Carrot is a cool-season crop that demands specific growing conditions and effective use of nitrogen (N) and water applications for successful commercial production. N and water management in carrot is crucial for increasing crop productivity and decreasing costs and nitrate leaching losses. The N needs of carrots for optimum storage root yield depends on the climate, soil texture and conditions, residual soil N from the previous season and irrigation management. There is not enough research on N management to free local growers of the worry associated with being short on the amounts applied, which may cause a loss in yield and profitability. The industry needs reliable information on N and consumptive water use of carrots to optimize irrigation and N management, enhance water and nitrogen use efficiency and achieve full economic gains in a sustainable soil and water quality approach.
This study aimed to quantify optimal N and water applications under current management practices and to fill knowledge gaps for N and water management in carrots through conducting experimental trials in the low desert of California. This article presents some of the information developed for desert fresh market carrots.
Field Trials and Measurements
Field trials were conducted on fresh market carrot cultivars at the UC Desert Research and Extension Center (DREC) and four commercial fields in the low desert region during the 2019-20 and 2020-21 seasons (Table 1). The sites represent various aspects of nitrogen applied (N applications ranged between 176 and 272 lbs ac-1), irrigation water applied (varied from 1.6 to 2.9 ac-feet/ac), irrigation systems (three fields under sprinkler irrigation and two fields under furrow irrigation) and soil types (sandy loam to silty clay loam).
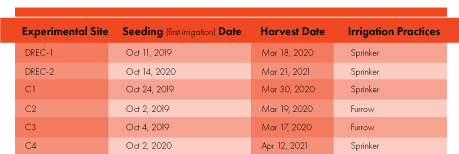
The DREC trials consisted of two irrigation regimes and three nitrogen scenarios (Fig. 2). At the commercial sites, due to logistical limitations, the measurements were carried out from five sub-areas selected (50 feet x 50 feet) in an experimental assigned plot (400 feet x 400 feet) with a homogeneous soil type, which was the dominant soil at the site. These areas represented common irrigation and N fertilizer management practices followed by growers.

The actual consumptive water use (actual crop evapotranspiration (ET)) was measured using the residual of the energy balance method with a combination of surface renewal and eddy covariance equipment (fully automated ET tower, Fig. 3). As an affordable tool to estimate actual crop ET, Tule Technology sensors were also set up at all experimental sites. The Tule ET data were verified using the ET estimates from the fully automated ET tower. Canopy images were taken on weekly to a 15-day basis utilizing an infrared camera (NDVI digital camera) to quantify crop canopy coverage over the crop season. Actual soil nitrate content (NO3-N) at the crop root zone (one to five feet) and the total N percentage in tops and roots were determined pre-seeding, post-harvest and monthly over the season. Plant measurements were carried out on 40-plant samples collected randomly per replication of each treatment/sub-area, and determinations were made on marketable yield and biomass accumulation. Fresh weight and dry weight of roots and foliage were measured on a regular basis.

Findings and Recommendations
Irrigation Management
The common irrigation practice in carrot stand establishment in the low desert is to irrigate the field every other day using sprinkler systems during the first two weeks after seeding. Carrots germinate slowly, and hence, the beds need to be kept moist to prevent crusting. A comparison between the averages of applied water and actual consumptive water use for a 30-day period after seeding suggested that carrots are typically over-irrigated during plant establishment. An average of 3.8 inches was measured as actual consumptive water use for this period across the experimental sites (Fig. 4), while the applied water varied from two to three times of this amount.
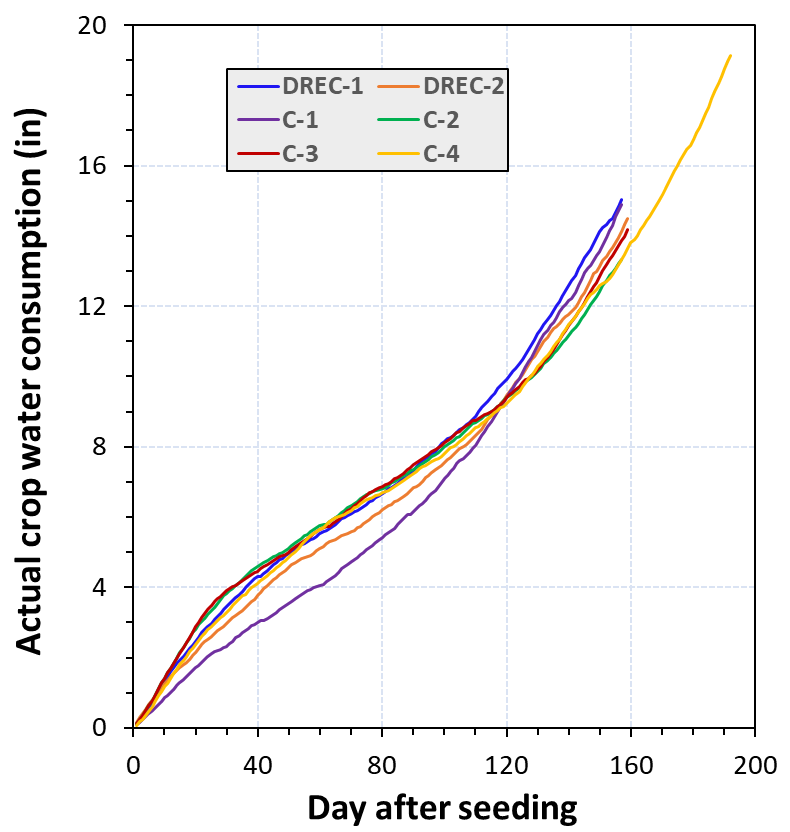
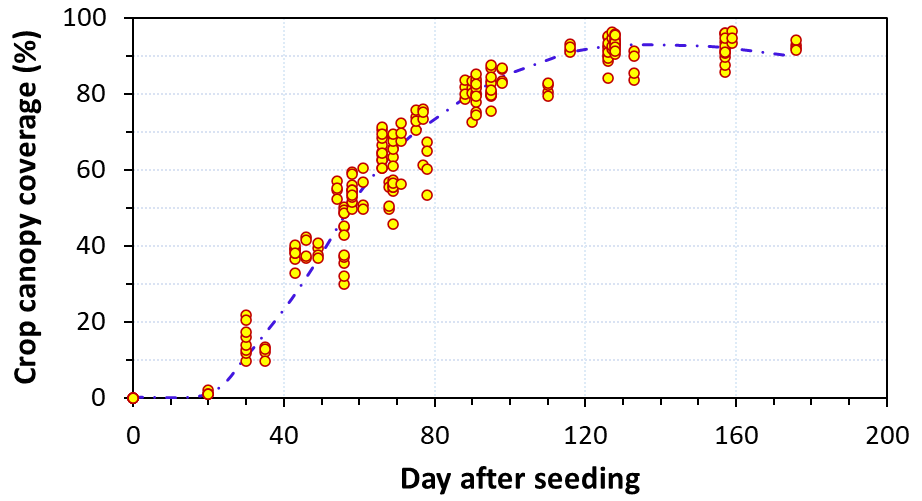
The results clearly demonstrated that the carrot sites had variable actual consumptive water uses depending upon early/late planting, irrigation practice, length of crop season, soil type and weather conditions. For instance, site C-4 was a sprinkler irrigated field with a dominant soil texture of sandy clay loam where the carrots were harvested very late 193 days after seeding (DAS). The seasonal consumptive water use was 19.2 inches at this site (Fig. 4). Our results show that the seasonal crop water use of fresh market carrots is nearly 16.0 inches for a typical crop season of 160 days with planting in October. Approximately 50% of crop water needs occurred during the first 100 days after seeding and the other 50% during the last 60 days before harvest. Crop canopy model developed in this study demonstrated that fresh market carrots reach 85% canopy coverage by 100 days after seeding.
The amount of water that needs to be applied in an individual field depends on crop water requirements and the efficiency of the irrigation system. Assuming an average irrigation efficiency of 70%, the approximate gross irrigation water needs of carrot fields in the low desert would be 2.0 ac-feet/ac (pre-irrigation is not included) for a 160-day crop season. Pre-irrigation along with proper irrigation scheduling over the season may effectively maintain crop water needs and salinity in carrots.
Water stress should be avoided throughout the carrot growing cycle. The critical period for irrigation is between fruit set and harvest. Sprinkler irrigation may be considered as a more effective irrigation tool when compared with furrow irrigation. More frequent and light irrigation events are possible by sprinkler irrigation. Over-irrigation of carrot fields increases the incidence of hairy roots, and severe drying and wetting cycles result in significant splitting of roots. Sprinklers also reduce salinity issues which is important since carrots are very sensitive to salt accumulation.
Nitrogen Management
The results demonstrated that a wide range of N accumulated both in roots and tops at harvest (Fig. 6). For instance, a total N content of 312.9 lbs ac-1 was observed in a fresh market carrot field with a long growing season of 193 days, including 202.9 and 110.0 lbs N ac-1 in roots and tops, respectively. The total N accumulated in plants (roots + tops) was less than 265 lbs ac-1 in the other sites.
A linear regression model was found for the total N uptake in roots after 60 to 73 DAS without declining near harvest (Fig. 6). Small, gradual increases in N contents of roots were observed until about 65 DAS. This suggested that N begins to accumulate at a rapid rate between 65 and 80 DAS; however, the period of rapid increase could vary depending on early (September) or late (November) plantings. N uptake in tops increased gradually following a quadratic regression, and in most sites levelled off or declined slightly late in the season. Although the N accumulated in tops appeared to drop down or level off in most sites beyond 120 to 145 DAS, the N content decline occurred after DAS 155 at site C-4 with a longer growing season.
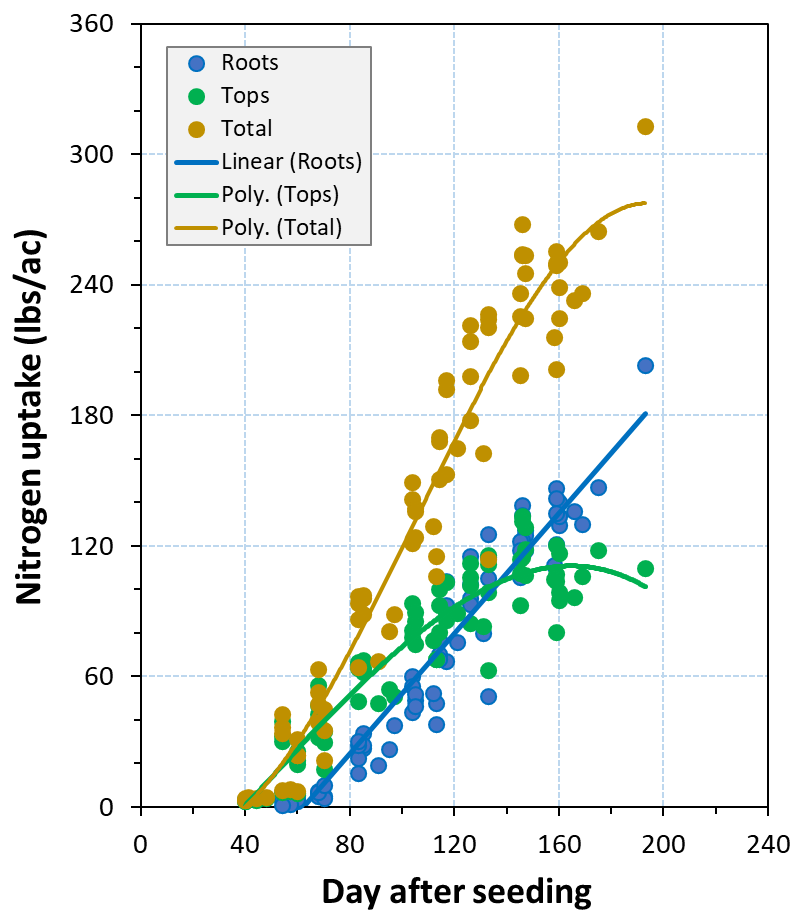
These findings suggest that a total N accumulation of 260 lbs ac-1 occurred by 160 DAS, with 145 lbs ac-1 in roots and 115 lbs ac-1 in tops. Across all sites, nearly 28% of seasonal N accumulation occurred by 80 DAS (Fig. 6) when the canopy cover reached an average of 67% (Fig. 5). The large proportion of this N content was taken up during a 30-day period (50 to 80 DAS). The results also suggest that nearly 50% of the total N was taken up during a 50-day period (80 to 130 DAS). This 50-day period appears to be the most critical period for N uptake, particularly in the storage roots, when carrots developed the large canopy and the extensive rooting system. The majority of N is taken up during the months of December to February, and, hence, proper N fertility in the effective crop root zone is essential during this period. For a 160-day crop season, 22% of N uptake could be accomplished over the last 30 days before harvest.
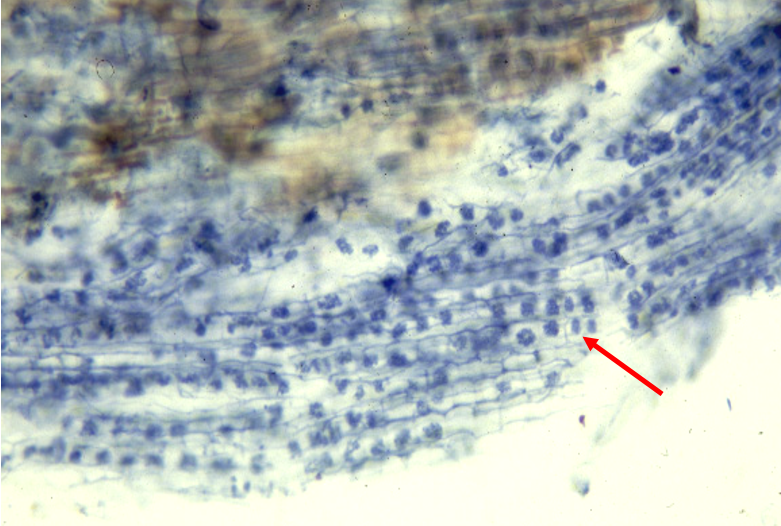
Carrots have a deep rooting system that allows for improved capture of N from deep in the soil profile. The fibrous roots were present at the depth of five feet below the soil surface at site DREC-2 (Fig. 7). There is a risk of leaching soil residual N due to heavy pre-irrigation (a common practice for salinity management in the low desert) in late summer prior to land preparation. N is likely accumulated at the deeper depths by the beginning of the growing season, and consequently, there is a potential N contribution from the soil for carrots when the roots are fully developed. Since residual soil N contribution can be considerable in carrots, pre-plant soil nitrate-N assessment down to 60 cm depth could be a tool enabling farmers to improve N management and maximize yield and quality while minimizing economic and environmental costs.

Careful management of N applications in the low desert carrots is crucial because fertilizers are the main source of N, particularly due to low organic matter content of the soils and very low nitrate level of the Colorado River water. Knowing this fact, the soil NO3-N contents pre-seeding and over the growing season at different sites revealed that none of the sites had N deficiency during the crop season, and consequently, the practice of splitting N applications, as done by the farmers (applying 9% to 15% of total seasonal N as pre-plant and the remainders through irrigation events over the season), was likely effective in most cases. It appears that the practice of 15% to 30% seasonal N applications though irrigation events 45 to 70 DAS has similar effectiveness to sidedress N applications.
Within the range of N application rates examined at the experimental sites, there were no significant relationships between carrot fresh root yield and N application rate, although the results suggested a positive effect of N application on carrot yield. Sufficient N availability in the crop root zone over the growing season and the lack of significant yield response to N applications demonstrate that N optimal rates could be likely less than the applied amounts in most sites. Adequate nitrogen and water applications reduce costs and help prevent leaching, while excess N may lead to excessive N storage in the roots, which may be a concern for processing carrots. Integrated optimal N and water management needs to be approached to accomplish greater N and water efficiency, and consequently keep lower rates beneficial to overall profitability.
Funding for this study was provided by California Department of Food and Agriculture (CDFA) Fertilizer Research and Education Program (FREP) and California Fresh Carrots Advisory Board.