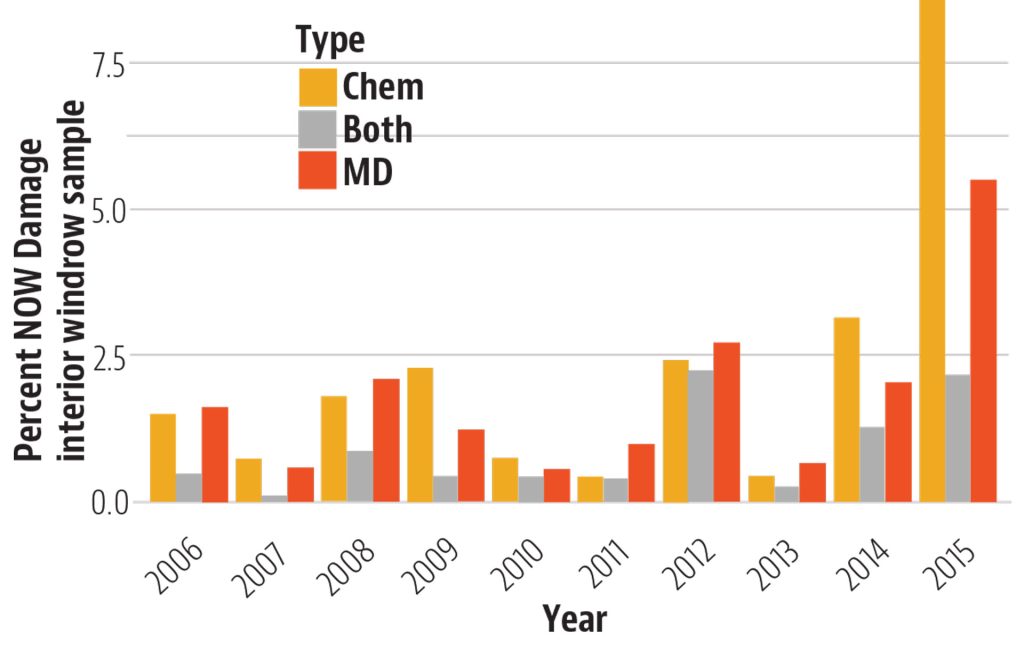
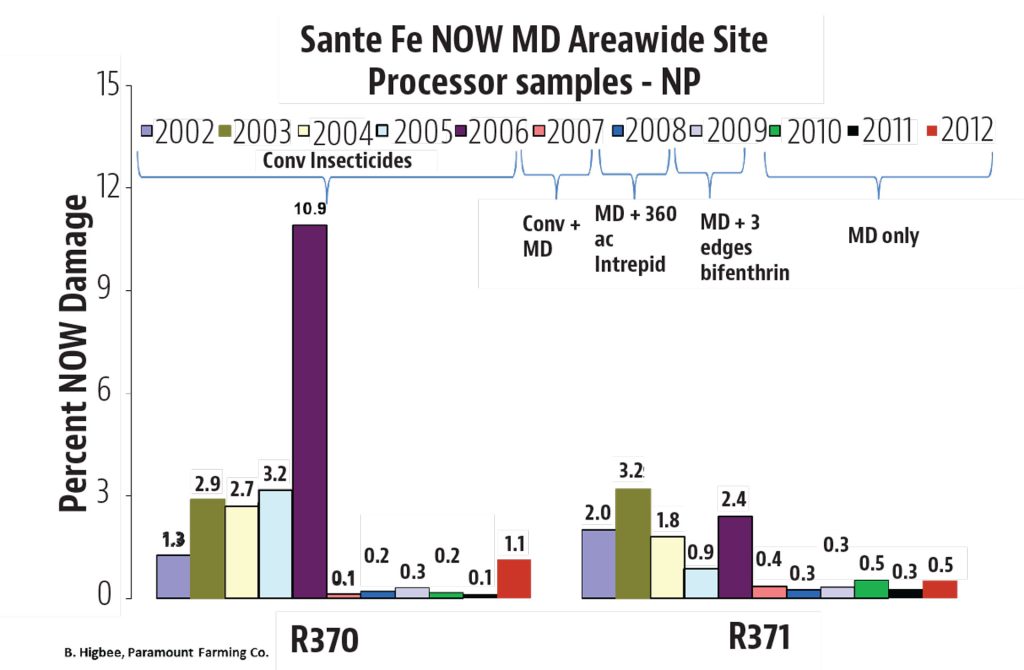
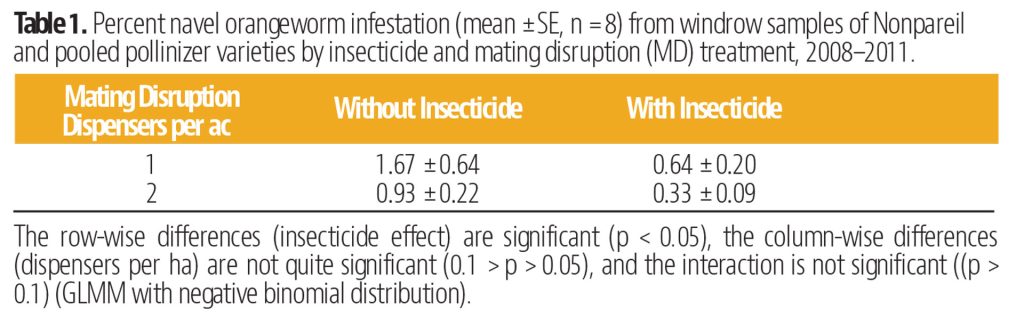
The Diamondback Moth (DBM, Plutella xylostella) is not a new insect pest by any means, but it has the capability to damage or destroy crops of tremendous value in a short time, and keeping up with viable management tactics can be a real challenge. This insect is present wherever cole crops (cabbage, broccoli, Brussels sprouts, etc.) are grown throughout the world. It can be a serious pest in canola, and while it does not prefer non-cruciferae crops, it has shown the capability to feed on other plant types, including legumes. Perhaps its most diabolical attribute is its ability to have up to 12 or more generations in a year, which gives them the potential to quickly become resistant to insecticides used against them.
Biology
The adult DBM is a small grayish moth that, when its wings are folded at rest, have dark markings, giving it the “diamondback” moniker. Eggs are deposited singly and are visible without a hand lens once a scout’s eyes have been trained to look for them. The larvae are a translucent green with spots and are easily distinguished from other caterpillars by their behavior of falling from plant surfaces when disturbed, often hanging from a silken thread. The larvae go through four instars before cocooning themselves to a leaf or stem to pupate into adults.
Damage and Control
Damage from DBM varies according to the age of the crop. Transplants carrying the eggs of DBM may be an initial source of infestation, but the adults are also known to travel long distances to find host plants. Young seedlings and transplants may have their growing tip chewed off, effectively killing or stunting the plant. Young larvae will strip off the outside layer of leaf tissue, leaving a “window pane” effect and harming the development of the crop. Older larvae will chew holes in mature leaves, which is especially damaging to cabbage. Later, as the larvae and the crop mature, there is the potential for damage to the crowns of broccoli and cauliflower, and larvae will burrow into maturing Brussels sprouts and cabbage heads. Reports have come from central Mexico, where a large amount of broccoli and other cole crops are grown, that up to 80% of a crop can be lost to diamondback moth damage. Should the genetics for diamide resistance become persistent for DBM, that mode of action which is the most recent may be rendered non-viable, and there are few modes of action other than peptides (Spear-Lep) coming online.

Control of diamondback moth relies mostly on the use of insecticides. There are no cole crops that have been modified to carry the Bacillus thuringiensis protein gene(s) that protects other crops, and for that matter, DBM has shown the ability to become resistant to Bt. There are natural enemies of diamondback moth, but they cannot be relied on to prevent economic damage to a crop. Insecticide resistance is a serious concern as DBM is capable of developing resistance to just about anything thrown at it, including the newest modes of action. Several companies have developed pheromone dispensers to disrupt mating of diamondback moth, and this is a potentially powerful tool to consider in a DBM management plan. An areawide management plan may prove difficult because of the adult’s ability to spread quickly and their ability to use weed species, especially mustards, as non-crop hosts.
Management Plan
A diamondback moth management plan should account for protection of seedlings/transplants by using a soil drench insecticide at planting and by inspecting plants for presence of DBM eggs. If cyazypyr was used at planting, be sure to note that so it or another member of that class (diamide) is not used again for seven to eight weeks.
Pheromone traps will alert a scout to the presence of DBM but will not be an indication of how severe the population could get or the correct timing for an application. Once the crop becomes established, twice-weekly scouting looking for eggs and early damage becomes necessary.
Rotation of insecticide modes of action is absolutely needed, taking into account the re-entry and pre-harvest intervals for each product. Bt insecticides (DiPel, Javelin, Xentari) are still considered effective unless otherwise noted by the local Extension office. The advantages of Bt are that it is non-toxic to anything except caterpillars and can be used close to harvest. The drawbacks are that they have a very short residual, do not have any effect on adults and cannot penetrate behind wrapper leaves where DBM larvae have burrowed to. It is also true of the newer chemistries that their modes of action target larvae only and will not control adult moths. Through diligence and effective treatments, damage from diamondback moth can be minimized.
Chemical and trade names used in this article do not constitute a recommendation. Consult a crop advisor, extension agent or manufacturer representative for more information.