Whether you’re harvesting nuts or fruit, all orchards have one thing in common at the end of their productive life cycles: Growers need to have a plan for tree removal.
Burning trees or selling wood chips to fuel-burning power plants is becoming a less viable option. Many power plants have gone out of business, and air regulations encourage or force growers to seek alternative disposal options.
Market shifts and policy changes have resulted in a relatively new concept: whole-orchard recycling (WOR). This regenerative agriculture practice encourages growers to use end-of-life orchards to improve organic matter and soil quality, water productivity and crop yield potential.
Yet, any grower who’s new to WOR will ask, “How much will this cost me?”, because the bottom line matters.
While more expensive than burning, there are many funding opportunities for growers who choose WOR. Plus, the long-term return on investment for this regenerative agriculture practice is well worth the outlay.
The Process: Recycle Your Orchard
Whole-orchard recycling turns low-yielding and unproductive trees into organic matter to support the next orchard. The process is relatively simple and straightforward.
Excavate the trees from the ground. Try to remove as much of the roots and the crown as possible from these aging and low-yielding trees.
Grind wood chips into either 2-inch or 4-inch screen size. 2-inch screen sizes are less likely to interfere with orchard floor management activities and less likely to harbor pathogens. They’ll also be easier to spread and incorporate into the soil.
Load wood chips into a modified manure spreader. This is the easiest way to disperse chips evenly across the orchard floor. Growers may consider 1-foot-deep dispersion of chips near piles, and 1-inch-thick dispersion in the far corners of the orchard.
Deep rip soil. A 5- to 6-foot-deep rip can break up compaction layers and pull out any remaining roots.
Smooth out ruts created by the ripper and incorporate chips. Growers can use a stubble disk, plow or rototiller to smooth out orchard floors and mix chips with soil.
Fumigate for extra protection. Experts suggest fumigating between 18 and 24 inches deep.
Almond Board of California, a leader in the WOR space, encourage growers to take a year off before replanting. Ripping, mulching, spreading, incorporating, fumigating and irrigating can take many months to complete.
Ways to Pay for the Upfront Cost of Whole-Orchard Recycling
The process is relatively simple, yet WOR is more expensive than traditional end-of-life management for orchards. Researchers at UC Davis estimated WOR can cost $125 to $810 more per acre than burning; yet, you don’t have to pay the bill on your own. There are many organizations that provide funding for this regenerative ag practice.
Here’s how to offset the up-front cost of WOR:
For Growers in the San Joaquin Valley: The Open Burning Incentive Program Growers in the San Joaquin Valley have a unique opportunity to apply for funding when opting to recycle their orchards. $220 million has been set aside for growers choosing WOR.
The funding grants $600 per acre to orchards and $1,300 per acre for vineyards.
For California Growers: California Department of Food and Agriculture Funding The California Department of Food and Agriculture’s Healthy Soil Initiative gave many growers funding for their no-till practices in their orchards and vineyards starting in 2021.
Keep an eye on their site for when applications open for 2024 funding.
For California Growers: Check with Your Local USDA-NRCS for Incentives Your local U.S. Department of Agriculture Natural Resource Conservation Service may provide incentives for WOR through the Conservation Stewardship Program.
For California Growers: Environmental Quality Incentive Program (EQIP)
Another program hosted by the USDA-NRCS, EQIP was created to provide financial and technical support to those using regenerative agriculture practices with direct ties to environmental benefits. WOR improves air quality and soil quality, so orchard owners qualify for funding.
Long-Term Return on Investment of Whole-Orchard Recycling
When you sort out the financing to recycle your orchard, you can look forward to the long-term benefits and return on investment. Here’s a short list of the perks WOR offers your future orchards.
Improves Soil Health and Structure by Adding Organic Matter to Soil
Burning releases carbon stored in woody debris. When this happens, carbon is lost forever and can’t support your future trees. Long-term and short-term studies suggest recycling trees can add carbon, soil nitrogen and organic matter. 22 to 40 tons of organic C is returned to the soil per acre when recycling trees.
This makes your unproductive trees a short-term and long-term carbon provider. Up to 30% of carbon can be released as CO2 in the first year after recycling. The rest slowly degrades, providing a continuous nutrient source for new orchards. Plus, when you incorporate wood chips into the soil, it enhances the soil structure and texture, improving tree root growth. Healthier soils have been found to dramatically improve the odds for a healthier orchard and higher yields.
Provides Better Carbon Sequestration As mentioned, burning trees releases carbon, but chipping and incorporating trees into your soil stores carbon. This can improve your community’s air quality and reduce greenhouse gas emissions.
Strengthens Water Productivity Since WOR is great for your soil, it’s also great for your water productivity. When trees are chipped and incorporated in the soil, it increases water use efficiency, infiltration, aggregation and water holding capacity.
While drought conditions have improved in California this year, growers would be smart to make the most of their water regardless of precipitation given that great water management can improve tree resilience.
Increases Cumulative Yield With healthy soil and improved water use, the health and productivity of an orchard increases, and the crop yield potential increases, too. One study at UC Davis found a second-generation almond orchard had a 20% increase in yield when WOR was coupled with regular nitrogen (N) and irrigation regime.
Pro Tip: Maximize Your Crop Yield Potential with a Carbon-rich Microbial Food Want to help your new trees access nutrients from your wood chips sooner? Maximize your WOR investment with a carbon-rich microbial food.
Start by conducting a test with a trusted crop adviser after tilling to find out the diversity and activity of your soil microbes. In most farm soils, 75% of soil microbes are inactive.
An active, healthy soil microbiome can:
- Improve soil texture and structure
- Improve water holding capacity by up to 10%
- Optimize fertilizer applications
- Optimize manure and compost applications
- Mitigate abiotic stress throughout the season
- Fight soilborne diseases
- Boost crop yield performance and ROI
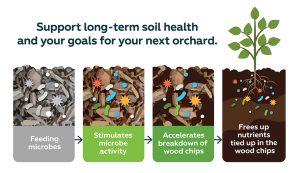
Feeding microbes stimulates microbe activity and accelerates breakdown of wood chips to free nutrients tied up in the wood chips.
By feeding your soil microbes a carbon product, you can make the most of the cost of WOR and support long-term soil health and your goals for your next orchard.
Community Benefits of Whole-Orchard Recycling
WOR is currently supported by air regulators and regenerative agriculture specialists, especially in areas of California like the San Joaquin Valley. There will be an 80% to 90% reduction in San Joaquin Valley orchard burning, according to CARB’s Chief of Air Quality Planning and Science Michael Benjamin.
- Last year, nut orchards with more than 500 total acres were unable to burn.
- This year, operations with more than 200 can no longer burn.
- By 2025, all ag burning will be banned except for special situations, like disease- or pest-ridden fields.
This is to support better air quality for the Valley. With over 9 million acres of farmland in California, burning will likely become less encouraged throughout the state year after year.
The times are changing, and those who find a way to make WOR work for them will reap the benefits in their crop yields to come. California growers will likely be on the forefront of this shift, continuing to produce tree crops that feed the U.S. By using the financial support available for WOR, growers can make the most of their aging orchards and support their future trees.