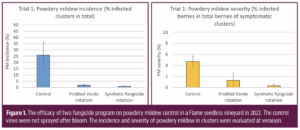
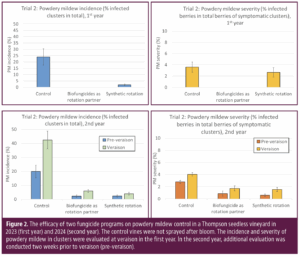
As a professional crop consultant, you leverage your education, training, experience and insights to assist your clients to improve their productive efficiency, mitigate production risk and thereby improve profitability of their businesses. But what about your business? You spend innumerable hours working in your business, but what do you spend working on your business?
For crop consultants, success extends far beyond the field’s edge. While expertise in agronomy, pest management and soil science is the foundation of the profession, a robust business plan is an essential framework that transforms technical knowledge into a thriving and resilient enterprise. A well-conceived business plan serves as a critical roadmap, guiding consultants from a mere practice to a professional operation, ensuring long-term viability and growth in an increasingly competitive agricultural landscape.
A comprehensive business plan does more than simply outline goals and objectives; it provides a clear and actionable strategy for achieving them. For a crop consultant, this blueprint is instrumental in navigating the unique challenges and opportunities of the industry, from the seasonality of work to the imperative of staying at the forefront of agricultural technology. It should be a living document, regularly reviewed and updated. Its core components should be tailored to the specific nuances of providing agronomic advice and services.

1. Defining Your Value Proposition: More Than Just Advice
At the heart of any successful business is a clear understanding of the value it provides. For a crop consultant, the value proposition goes beyond simple recommendations. It’s about demonstrating a tangible positive net return for the farm client. A strong business plan will articulate this clearly. Are you focused on maximizing yield, optimizing input costs, promoting sustainable practices or a combination of these? Your value proposition should clearly answer the question, “Why should a grower hire you over another consultant or even merely relying upon their own knowledge?” This section of the plan should detail the specific outcomes and benefits clients can expect.
2. Services Offered and Specialization
The business plan must meticulously outline the services provided. This could range from basic soil sampling and analysis to comprehensive, year-round crop management programs. Consider creating tiered service packages to cater to different farm sizes and needs. This section should also address any areas of specialization. Do you have expertise in a particular crop, irrigation management, precision agriculture technologies, regenerative agricultural practices or organic certification? Highlighting a niche can be a powerful differentiator in the market.
3. Market Analysis and Client Acquisition
A thorough understanding of the target market is crucial. The business plan should identify the types of farms and growers you aim to serve. What is the acreage, crop type and technological adoption level of your ideal client? Furthermore, this section needs a detailed client acquisition strategy. How will you reach potential clients? This could involve:
• Networking: Build relationships with local growers, agronomists and industry suppliers.
• Digital presence: Create a professional website and use social media to share valuable content and testimonials.
• Referrals: Develop a system to encourage referrals from satisfied clients.
• Thought leadership: Speak at local agricultural events or write articles for trade publications.
4. Operations and Technology
How will you deliver your services efficiently and effectively? The operational plan should detail your periodic activities, including scheduling, data management and reporting to clients. A critical component for the modern crop consultant is the integration of technology. Your business plan should outline your strategy for utilizing farm management software, drone technology, sensor data and other precision agriculture tools. This not only enhances the quality of your recommendations but also demonstrates a commitment to innovation.
5. Financial Projections and Strategy
A solid financial plan is the ultimate measure of a business’ health. This section should include:
• Startup costs: If you are just beginning, detail the initial investment required for equipment, software, insurance and marketing.
• Pricing structure: Clearly define your fees, whether they are on a per-acre, hourly or project basis.
• Revenue forecasts: Project your income based on your target number of clients and service packages.
• Expense budget: Account for all potential costs, including vehicle maintenance, software subscriptions, professional development and insurance.
• Cash flow management: Address the seasonality of income and plan for periods of lower activity.

The Tangible Benefits of a Well-Laid Plan
The effort invested in creating a comprehensive business plan yields significant returns for a crop consultant. It provides a clear sense of direction, transforming a passion for agriculture into a structured and profitable business. A well-articulated plan is also an invaluable tool for securing financing from lenders who want to see a clear path to profitability and risk management.
Ultimately, a business plan empowers crop consultants to be proactive rather than reactive. It allows them to anticipate market trends, identify new opportunities and make informed decisions that will not only benefit their own bottom line but also contribute to the success and sustainability of the growers they serve. In an industry defined by constant change, a solid business plan is the steady hand that guides the modern crop consultant toward a prosperous future.
But developing and implementing a comprehensive business plan is not commonly loaded into a crop consultant’s skills toolkit. How does a crop consultant get this mission accomplished or where can they acquire the needed skills? There is commercial interactive software available to assist in stepping through the processes. There are agricultural consultants that specialize in business development and business management. Check out the directory of the American Society of Agricultural Consultants. There are options for professional training in this area. A firm of which I am a co-founder (MACS Academy LLC) offers a course, Agricultural Consulting Practice Management, which covers business plan development among several pertinent topics. Choose the path that best fits you/your business.