Pierce’s disease is caused by the bacterium Xylella fastidiosa. These bacteria live within xylem, the vascular tissue through which water travels in a plant. As the bacteria population grows, it stimulates the plant to produce tyloses. The combination of bacteria and tyloses cause vessel plugging, which restricts water movement in the plant, thus causing many of the disease symptoms. These blockages will eventually lead to the vine’s death. It is estimated that Pierce’s disease costs the California grape industry $56.1 million a year in lost productivity (Tumber et al., 2014). To minimize losses, it is important to understand the biology of the disease, including the bacteria’s host range, how the bacteria moves from plant to plant, and how to identify infected plants will help growers prevent losses and control the disease.
About the Bacterium
The bacterium X. fastidiosa has a large known host range. The European Food Safety Authority maintains a database of known hosts for X. fastidiosa (their updated list approved in April 2020 can be found at doi.org/10.2903/j.efsa.2020.6114.) Research done throughout California has identified many hosts, including weeds such as shepherd’s purse (Capsella bursa-pastoris), filaree (Erodium spp.), cheeseweed (Malva parvifolia), burclover (Medicago polymorpha) and annual bluegrass (Poa annua) among many others (Shepland et al., 2006 and Costa et al., 2004). Overall, at least 350 host plants have been identified from over 75 plant families as hosts for X. fastidiosa. From a control standpoint, once X. fastidiosa has been introduced to a geographic area, it will be virtually impossible to eliminate it from that location with such a wide variety of possible hosts.
X. fastidiosa does have another level of complexity. To date, four distinct subspecies of X. fastidiosa have been identified. X. fastidiosa ssp. fastidiosa is the subspecies that causes Pierce’s disease in grapevine, while X. fastidiosa ssp. multiplex is the subspecies that causes almond leaf scorch (Rapicavoli et al., 2018). This does mean that almond trees with almond leaf scorch would be unable to be the source of Pierce’s disease in a vineyard. To narrow down the definitive host range, grape-specific PD (X. fastidiosa ssp. fastidiosa) was inoculated into a range of possible host plants using glassy-winged sharpshooters as a vector. After an incubation period, multiple positive ELISA results were obtained for several plants including black mustard (Brassica nigra), black sage (Salvia mellifera), mirror plant (Coprosma repens), Spanish broom (Spartium junceum), Mexican elderberry (Sambucus mexicana), almond (Butte) (Prunus dulcis), white sage (Salvia apiana), sycamore (Platunus racemose) and coast live oak (Quercus agrifolia), confirming that they could host the bacteria (Costa et al., 2004). This does ultimately lower the number of possible plant hosts for X. fastidiosa ssp. fastidiosa; however, it still includes a long list of common plants found in and around vineyards, enough that even including this reduced host list, managing only these species, and other hosts yet to be identified, is still outside the ability in most growing regions.
Insect Vectors
Bacteria within the xylem tissue of one plant may be spread to another plant through the feeding activities of certain xylem-feeding insects. In vineyards, two groups of insects have been identified as possible vectors: sharpshooters and spittlebugs. Spittlebugs have been shown to vector X. fastidiosa in controlled settings, but their importance as a Pierce’s disease vector in vineyards is unclear. Sharpshooters, on the other hand, are known to be effective vectors of Pierce’s disease in vineyards.
There are several different sharpshooters in California that vector X. fastidiosa. The most important of these in the coastal portions of California is the blue-green sharpshooter. This sharpshooter is not adapted to the hotter climate of the San Joaquin Valley (SJV). In the SJV, there are three other sharpshooters: the green sharpshooter (Draeculacephala minerva), the red-headed sharpshooter (Xyphon fulgida) and the glassy-winged sharpshooter (Homalodisca vitripennis).
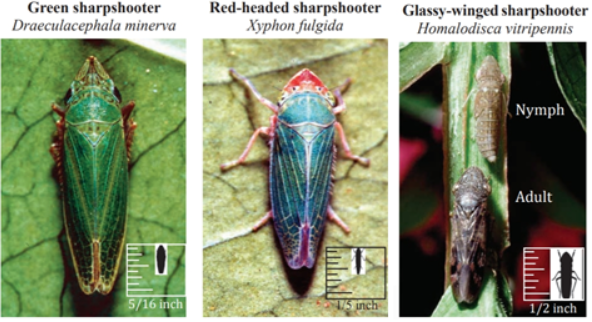
Green and Red-Headed Sharpshooter
The green sharpshooter and the red-headed sharpshooter are both small and prefer to feed on grasses. The red-headed sharpshooter is specifically drawn to and reproduces on Bermudagrass. Both the green and red-headed sharpshooter can be found in irrigated pastures and along waterways such as streams, creeks, canals and ditches. Neither of these sharpshooters prefers to feed on grapevines, however they may do so under certain conditions and thus transmit Pierce’s disease. Since they don’t prefer grapevines, they tend not to spread deeply into vineyards; thus, when these vectors transmit Xylella, it is usually only to grapevines along the edges of a vineyard, whereas vines in the middle or the sides away from the green or red-headed sharpshooters’ preferred habitat are not affected.
Glassy-Winged Sharpshooter
The glassy-winged sharpshooter is twice the size of either of the other two sharpshooters. Their large size makes them more effective as a vector for Pierce’s disease because they can travel further than smaller sharpshooters and feed more effectively on a wider variety of plants, including woody plants such as grapes. To date, over 350 plants have been identified as hosts of glassy-winged sharp shooter (cdfa.ca.gov/pdcp/Documents/HostListCommon.pdf). Many of the hosts for glassy-winged sharpshooters are also hosts for X. fastidiosa. One of the key hosts for both glassy-winged sharpshooters and X. fastidiosa in the SJV, and for local control of Pierce’s disease, is citrus. The large feeding range of the glassy-winged sharpshooter also means that it can spread the disease throughout the vineyard instead of just to the edges.
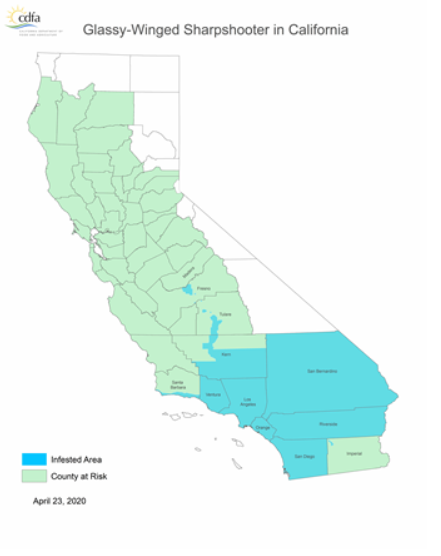
The glassy-winged sharpshooter is not a native California insect, only arriving in California in the late 1980s (first recorded in 1989). As this non-native pest is such a dangerous vector, the CDFA tracks their distribution. Most of Kern county, parts of Tulare and Fresno counties, and a very small sliver of Madera county just over the San Joaquin River are all hosts to naturalized populations of glassy-winged sharpshooters within the SJV. In SoCal, Ventura, Los Angeles, Orange, San Bernardino, Riverside and San Diego counties as well as portions of Santa Barbara county and a small section of Imperial county all play host to endemic populations.
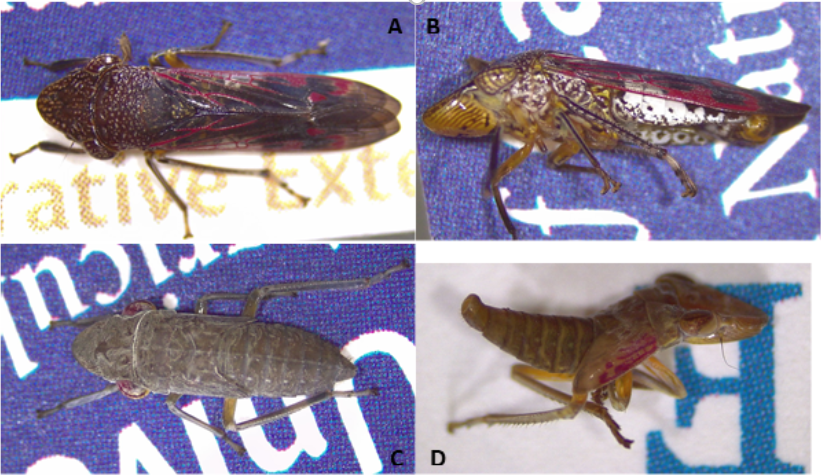
Identification of glassy-winged sharpshooters within and near these areas is important for controlling their spread as well as the spread of Pierce’s disease. At the top, the insect has a deep brown color with creamy white dots on the head and thorax. These colors and dots continue onto the abdomen; however, here they are covered with transparent wings (the source of their glassy name). Highlighting the glassy wings are red lines and patches which can be seen from both the top and side.
The other main identifying mark is the flat white marking along the side of the of the abdomen. When sitting on a stem, this white mark stands out under and through the wings of this sharpshooter. Younger nymph glassy-winger sharpshooters have yet to develop their namesake wings. Their bodies are a lighter grayish-brown with very small white dots. In this stage, the standout feature is their red eyes. The red is the same color that will soon highlight the parent’s wings.
Later-stage nymphs have started to transition to the adult body color, and the red color in the eye is mostly lost. However, the red color has transitioned onto the wing pads in a pattern that has started to develop the adult wing’s patterning.
Vector Monitoring and Treatment
Monitoring for glassy-winged sharpshooters can be done using yellow sticky cards. It is recommended to use cards that are at least 5.5” x 9” in size. One card should be placed for every 10 acres and checked weekly for recent activity. Monitoring should be done from budbreak through November. If a glassy-winged sharpshooter is found, and you are outside of a known population center, please contact your local agriculture commissioner’s office or cooperative extension office. Green and red-headed sharpshooters are not attracted to yellow sticky cards, so to monitor their populations you will need to use a sweep net. Sweep lush green grasses near and within your vineyard in April and May to assess population size.
For both green and red-headed sharpshooters, finding two adults in 50 sweeps warrants a response. Unfortunately, as both sharpshooters are only incidentally on grapevines, treating the grapevines will not help the situation. The preferred habitat (lush grassy areas) will need to be addressed. Due to the overlapping generations seen in these sharpshooters, insecticide treatments are often ineffective. Removal of preferred habitat is a more effective treatment option for these sharpshooters.
For glassy-winged sharpshooters, a single find warrants a response. A list of treatment options for glassy-winged sharpshooters can be found on the UC IPM webpage at ipm.ucanr.edu/PMG/r302301711.html. The most common insecticides used for glassy-winged sharpshooter control contain the active ingredient imidacloprid. As with all chemical control, it is important to rotate active ingredients regularly.
Research conducted in 2017 showed that several other insecticides had long-term control of glassy-wing sharpshooters. These included Sivanto (a.i. Flupyradifurone) and Assail (a.i. Acetamiprid), which still showed greater than 90% mortality 7 weeks after application; Actara (a.i. Thiamethoxam), which still showed greater than 90% mortality 5 weeks after application; Harvanta (a.i. Cyclaniliprole), which still showed greater than 90% mortality 4 weeks after application; and Sequoia (a.i. Sulfoxaflor), which still showed greater than 90% mortality 3 weeks after application (Haviland and Rill 2019). This research was conducted in citrus because relying on vineyard-only management is not enough for glassy-winged sharpshooters. With the larger range of the glassy-winged sharpshooter, it is important to focus on an area-wide approach. A pilot program with cooperation between grape and citrus growers has shown great promise in Kern county. Citrus groves are a primary overwintering spot for glassy-winged sharpshooters. When treatments can be applied to these locations, it can lower the number of glassy-winged sharpshooters and, thus, the presence of PD in the area.

Vine Infections
Early identification of infected vines is the final step in preventing a larger problem from Pierce’s disease. Infected vines can be a source of the disease for vectors to spread to neighboring vines. They are also a strong indicator that the bacteria and a vector are present in your location.
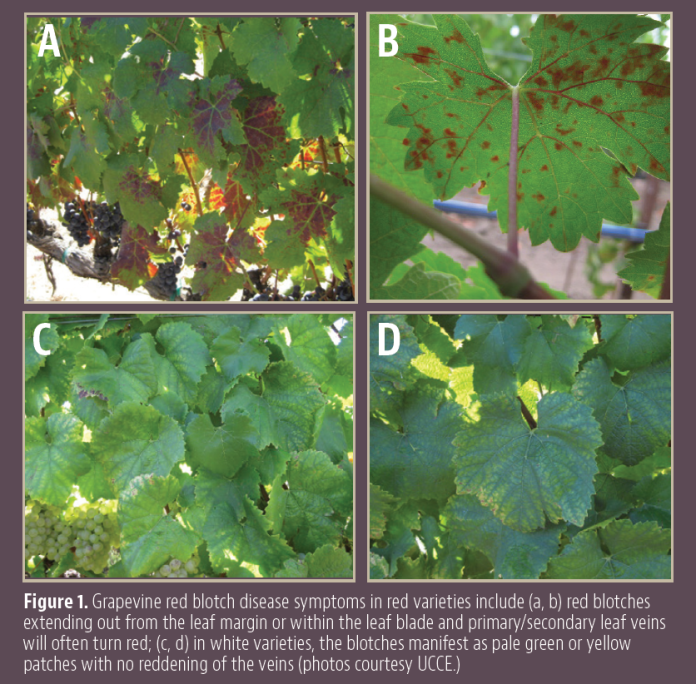
The leaves of infected vines will turn yellow (for green varieties) or red (for red varieties) along the margins. This discoloration will then work inwards from the margin, with the discoloration quickly turning to brown/dried dead tissue. This often happens unevenly or in sections. Shoot tissue also shows an uneven maturation process, leaving green islands within lignified brown tissue.
Affected leaves eventually fall off, but will sometimes leave the petiole still attached to the shoot. Not all these symptoms will be found on every infected vine. If you suspect a vine is infected with Pierce’s disease, you can contact your county’s viticulture advisor for corroboration. Ultimately, a diagnostic analysis is required to confirm the presence of X. fastidiosa in the suspected vine. Table 1 lists laboratories within California that offer Pierce’s disease testing.
References
Costa, H. S., Raetz, E., Pinckard, T. R., Gispert, C., Hernandez-Martinez, R., Dumenyo, C. K., and Cooksey, D. A. 2004. Plant hosts of Xylella fastidiosa in and near southern California vineyards. Plant Dis. 88:1255-1261.
Haviland, D. and Rill, S. 2019 Evaluation of glassy-winged sharpshooter mortality following exposure to aged insecticide residues, 2017. Arthropod Management Tests, 44(1), 1–1. doi: 10.1093/amt/tsz075
Rapicavoli, J., Ingel, B., Blanco-Ulate, B. Cantu, D. and Roper, C. 2018. Xylella fastidiosa: an examination of a re-emerging plant pathogen. Mol. Plant Pathol., 19(4), 786–800
Shapland, E. B., Daane, K. M., Yokota, G. Y., Wistrom, C., Connell, J. H., Duncan, R. A., and Viveros, M. A. 2006. Ground vegetation survey for Xylella fastidiosa in California almond or orchards. Plant Dis. 90:905-909.
Tumber, K. P, Alston, J. M, & Fuller, K. 2014. Pierce’s disease costs California $104 million per year. California Agriculture, 68(1-2)