Sprinkler and flood irrigation often generates runoff that transports sediment from agricultural fields to downstream rivers, lakes, and estuaries. Additionally, some classes of pesticides, such as pyrethroids, bind to the suspended sediments in tail water which can cause toxicity to aquatic organisms in these receiving waters. Currently numerous rivers and creeks in California are considered impaired by pesticides and sediment transported with drainage from agricultural land. As water quality regulations become stricter, growers will need to implement practices on their farms that treat potential pollutants in runoff.
Tail water can be a particularly challenging water quality problem on the central coast of California where overhead sprinklers are widely used for vegetable production. Sprinklers can contribute to high concentrations of suspended sediment in tail water because the force of the falling water droplets erode soil aggregates and allow fine particles to be carried with runoff water. Research that we have conducted on the central coast has shown that adding a low concentration of polyacrylamide to irrigation water can dramatically reduce sediment loads and sediment-bound pesticides in agricultural tail water (Fig. 1). Across a range of soil types, polyacrylamide treatment reduced sediment concentration in runoff by more than 90 percent on average. On some highly erosive soils polyacrylamide reduced sediment concentration in sprinkler induced runoff by more than 98 percent. Total phosphorus and nitrogen concentrations in sprinkler runoff were also reduced by 40 percent to 70 percent using polyacrylamide.
Despite dramatic improvements in water quality, polyacrylamide, also called PAM, has been slow to be adopted as a management practice on the central coast and in much of California. One reason may be because of misunderstandings about how to most effectively use PAM for treating runoff, especially for sprinkler irrigation. Another reason is that several of the physical properties of polyacrylamide make it challenging for handling and applying to fields.

Brief background on PAM
Polyacrylamide (PAM) is a simple polymer molecule made of carbon, nitrogen, and oxygen. Various forms of PAM exist, but the type used to stabilize soil and prevent erosion is a very large, mostly negatively charged molecule (12-15 megagrams per mole). Agricultural PAM is commercially available in dry powder (granular), emulsified liquid, and dry tablet forms, and costs as little as $4 to $6 per pound of active ingredient depending on the formulation, supplier, and cost of the raw materials used for manufacturing PAM (i.e. natural gas). PAM is used for many nonagricultural purposes such as a flocculant for waste and potable water treatment, processing and washing of fruits and vegetables, clarification of juices, and paper production. It is also a component of makeup.
Use of PAM for Irrigation and Erosion Control
Because PAM is a very long, linear molecule it easily binds to soil aggregates, thereby preventing soil erosion during irrigation events. Beginning in the early 1990’s numerous studies demonstrated that low application rates of PAM (1 to 2 lb/acre) reduced runoff and improved water quality in furrow systems by stabilizing the aggregate structure of soil, improving infiltration, and flocculating out suspended sediments from irrigation tail water. Most of the research and demonstrations of PAM were conducted in furrow systems in Idaho and Washington states where soils are very erodible. By 1999, almost 1 million acres of land were annually treated with PAM in the northwest of the United States. Additionally, growers in the San Joaquin Valley and the Bakersfield areas of California used PAM to reduce soil erosion under furrow and flood irrigation.
Working with PAM
PAM can be very difficult to use if it is not handled correctly. Wet PAM is very slippery, and because it solubilizes slowly in water, PAM spills should be cleaned up with a dry absorbent rather than washing it with water. Although it is not toxic to humans, some precautions should be taken when handling PAM: Use gloves to avoid irritation to skin. Goggles will prevent eye exposure. Also, a dust mask is recommended when pouring or handling dry granular or powder forms of PAM to avoid inhalation.
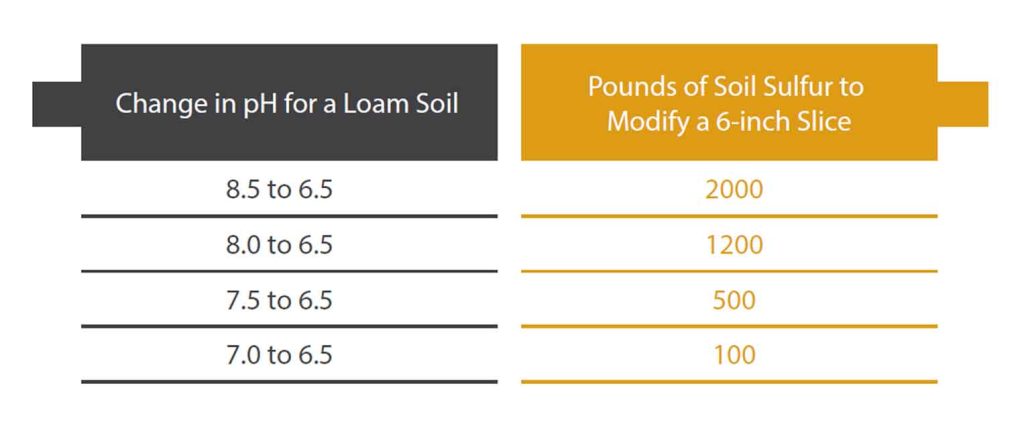
One rule of thumb to keep in mind is that it is much easier to add water to PAM than to add PAM to water. Dry PAM rapidly absorbs water, increasing its original volume many times to become a slimy, gooey substance. Dissolving dry PAM in water can be challenging. Because PAM is a very large molecule it does not dissolve readily into water and requires many hours of agitation to dissolve. It will often stick to the side of a tank when being mixed. Also, mixing up concentrations greater than 0.15 percent in water is nearly impossible because the solution becomes very viscous and difficult to agitate. Some manufacturers sell effervescent PAM tablets which aids dissolution in water, but still only relatively dilute solutions can be mixed up.
For these reasons it easiest to use liquid PAM products that have been emulsified with a carrier such as mineral oil or humectants, or work with dry PAM products, such as granular PAM or PAM in a tablet form. The emulsified liquid products generally have active ingredient concentrations ranging from 25 to 50 percent.
Application Methods
For applications in furrow systems dry or liquid product can be added to water flowing in a head ditch or main line (if gated pipe is used) at a rate to achieve a 2.5 to 10 ppm (parts per million) concentration. Automated equipment can be used to spoon feed granular PAM into flowing canal water. The application can be made continuously during the irrigation or until the water advances almost to the end of the furrows. An alternate application method, called the “patch method” involves spreading granular PAM to the first 3 to 5 feet of each furrow. The granular PAM slowly dissolves as water flows down the furrows. A similar strategy is to add a PAM tablet at the beginning of each furrow. Applications into sprinkler systems require specialized equipment for injecting either liquid PAM or dry PAM into pressurized pipe which will be discussed in more detail later.
Environmental and Food Safety
Only PAM products labeled for application to food crops should be used in agriculture. Also, the buyer/processor/shipper of the produce should be informed that PAM is being applied to the crop, especially if the application is made near harvest.
Agricultural PAM used for soil erosion is not toxic to mammals. Environmental studies of anionic (negatively charged) PAM have not shown any detriment to fish, algae, and aquatic invertebrates such as Ceriodaphnia dubia, and Hyalella azteca. Polyacrylamide is sometimes confused with acrylamide monomer, a precursor in the manufacturing of PAM. Acrylamide monomer, a potent neurotoxin, has a high, acute toxicity in mammals. The Federal Environmental Protection Agency (EPA) requires that PAM sold for agricultural uses contain less than 0.05 percent acrylamide monomer. In soil, PAM degrades rapidly by physical, chemical, biological, and photochemical processes, but it does not decompose into the acrylamide monomer. A previous study of the movement of PAM from agricultural fields showed that less than three percent of the applied product remained in the runoff leaving the field. The remaining PAM in the tail water was almost completely removed through adsorption to suspended sediments as the water flowed a distance of 300 to 1000 ft in the tail water ditch.
One concern with using emulsified liquid PAM is that the mineral oil carrier can have toxicity to downstream aquatic invertebrates. However, toxicity from the mineral oil can be avoided by using PAM formulations with either high concentrations of PAM (>50 percent active ingredient) or with non-oil carriers such as humectants.
Optimizing PAM for Sprinklers
Although many research studies have evaluated the efficacy of PAM in furrow systems, fewer studies have evaluated the use of PAM with sprinklers. Applications of PAM made before irrigating with sprinklers, such as by spraying PAM solution or broadcasting dry product on the surface of the soil were far less effective than continuously injecting PAM at a low rate into the irrigation water. Injecting PAM only at the beginning of an irrigation was also less effective in controlling sediment in runoff than a continuous application at a low concentration during the entire irrigation. Our studies on the central coast show that injecting PAM to achieve a 5 ppm concentration in the irrigation water provided the highest reduction in sediment, nutrients, and pesticides in the tail water using the least amount of product. In some fields 2.5 ppm PAM provided equal efficacy for control of suspended sediments as 5 ppm PAM. Treatment with PAM should be started with the first irrigation after planting and continue during the following two to three irrigations. PAM should be reapplied when sprinkler irrigating after the field has been cultivated. Product can be saved in fields where very little runoff occurs during the first few hours of an irrigation by making an initial application for the first half hour and then applying product again when runoff becomes significant.
Injecting PAM into Pressurized Irrigation Systems
The chemical characteristics of PAM that make it so effective as a flocculant, also make it difficult to inject into pressurized irrigation systems. Liquid PAM solutions are viscous and will clog chemigation equipment with valves such as diaphragm and piston pumps, as well as venturi injectors. Also, because the desired PAM concentration in the irrigation water is low, injection rates as low as one to three ounces per minute are needed to treat 10 to 15-acre fields. Most small, gas-powered centrifugal pumps usually cannot be easily calibrated to inject at these low rates. Peristaltic pumps can be adjusted to inject slowly but often are not designed to operate under high pressures that are common in sprinkler main lines. The best type of pump that we have identified for injecting liquid PAM is an auger pump (Fig. 2.) which has no valves and can inject viscous solutions at very low rates. These pumps also maintain a consistent injection rate at pressures as high as 100 psi (pounds per square inch).

Although liquid formulations of PAM are the best option for pressurized irrigation systems, we are currently exploring methods of injecting dry PAM into pressurized sprinkler systems. The advantage of dry PAM is that it is generally cheaper than liquid products and the possibility of introducing toxicity from the inactive emulsifying ingredients in the liquid products is eliminated. Another advantage of this approach is that it may require less labor since no pump must be calibrated and managed during an irrigation. The dry PAM applicator is loaded with cartridges containing either granular or tablet forms of PAM (Fig 3.). A portion of the water from the main line is diverted through the applicator chambers and then added back to the main water stream. Although the PAM concentration is lower than can be achieved with liquid PAM, preliminary tests have shown that as much as 90 percent of the suspended sediments can be eliminated in the runoff (Fig 4). Further studies during the upcoming season will evaluate the practicality of use this applicator in commercial fields.

Other Potential Benefits of PAM
In addition to water quality benefits, we have observed or measured agronomic benefits from the use of PAM. Because PAM stabilizes soil aggregates, soil is less likely to crust under the impact of sprinkler droplets, which improves infiltration and decreases the volume of runoff. In one field trial, PAM reduced runoff from four successive sprinkler irrigations from 4000 gallons per acre to less than 1500 gallons per acre. Less crusting of the soil may also improve germination of small seeded vegetables such as lettuce. In one of four replicated field trials conducted in commercial lettuce fields, we were able to measure an increase in yield and plant weight with the use of PAM. This yield increase may be a result of better infiltration of moisture or because the seed emerged earlier than in the non-treated plots. Although we have not conducted long-term studies, anecdotal reports from growers who applied PAM to their fields over successive years were that soil structure was improved by keeping the fine particles in place.

An additional benefit of PAM is saving costs associated with cleaning out ditches and retention ponds that become clogged with sediment during the irrigation season. Often once or twice per year growers on the east side of the Salinas valley who farm on soils prone to crusting and runoff must schedule a backhoe and crew to remove sediment from ditches and ponds and redistribute the material in their fields. To reduce costs with using PAM, growers also can receive cost-share payments under the United States Department of Agriculture (USDA) Natural Resources Conservation Service (NRCS) Environmental Quality Incentive Program) (EQIP).
In summary here are a few key concepts on using PAM for controlling sediment in runoff:
- Polyacrylamide is a long linear molecule that binds to soil and can flocculate suspended sediments in water.
- PAM does not readily solubilize in water and increases the viscosity of water (thickens).
- Concentrations of 2.5 to 5 ppm PAM in irrigation water are ideal for optimizing erosion control benefits under sprinkler and furrow irrigation.
- For agricultural purposes only use anionic PAM products for erosion control and labeled for use on food crops.
- Small amounts of granular PAM can be applied to the beginning of furrows before flood irrigating (1 to 3 lbs/acre).
- For sprinklers PAM needs to be injected into the irrigation water during the entire irrigation event.
- Applying PAM to the soil before sprinkle irrigating or only at the beginning of an irrigation will not maximize control of sediment in runoff.
- PAM should be applied during the first three to four irrigations after planting or transplanting and when irrigating after soil cultivation.
- Auger pumps are ideal for metering liquid PAM products into pressurized sprinkler water.
- PAM itself is not toxic, but the mineral oil in some liquid PAM products can be toxic to aquatic organisms.
- A prototype applicator is being developed to inject dry PAM into pressurized sprinkler systems, although it is not yet commercially available.
Further information on using polyacrylamide is available on the UC Cooperative Extension Website for Monterey County (http://cemonterey.ucanr.edu/Custom_Program567/Polyacrylamide_PAM/)