Increase yield potential and maintain a healthy orchard by selecting the right crop protection solutions for your unique needs. Prevent disease, eradicate tough weeds and control insects with branded-generic fungicides, herbicides and insecticides from Atticus. Explore the broad and expanding tree nut product portfolio for season-long protection. Learn more at Atticusllc.com
Wilt Disease Expanding in Strawberry Production

Wilt disease in strawberries caused by the pathogen Fusarium oxysporum forma speciales fragariae is expanding quickly and merits attention from strawberry growers.
Mark Bolda, UCCE strawberry and caneberry farm advisor in Santa Cruz County, emphasized that fumigation operations in infected fields are worth the cost even when using plants resistant to this pathogen. Speaking at the 2021 Crop Consultant Conference, Bolda also noted that crop termination works to improve yields in resistant varieties through the season and in the first half of the season in susceptible varieties.
“Crop termination very much looks like it enhances chloropicrin efficacy in susceptible varieties,” Bolda said.
The majority of the genus Fusarium oxysporum is not pathogenic and is common in soil and around roots. Individual types are specific to host plants. This pathogen does grow on other field crops, but does not thrive, except on strawberries. Infected plants become noticeable from May to June as crowns split and become discolored. Bolda said 80% of the disease load is in the crown of the plant.
Bolda said Fusarium in strawberry could possibly coincide with the reduction and prohibition of methyl bromide. When many plants are diseased at once in a field, that does not necessarily mean the disease came with the transplants. This specific pathogen does not show symptoms at low levels and can augment populations over several successive crops of strawberries. Management of this pathogen consists of sanitation, fumigation, crop rotation and possibly fungicide applications.
He advised minimizing movement of soil from field to field on shoes and equipment to keep from spreading the pathogen to uninfected fields. Crop rotation is at least 18 months before returning to strawberries. The longer the better, Bolda said.
Bolda’s field trial set out to test efficacy of fumigant treatments and crop rotations against the strawberry-specific Fusarium pathogen. Treatments tested were KPAM at 20 gallons per acre and crop termination, Dominus at 20 gallons per acres, Tri Clor 80 at 350 pounds per acre and KPAM drip at 47 gallons per acre. Varieties were Fusarium-resistant San Andreas, Fusarium-susceptible Monterey and Fusarium-resistant Fronteras. Dominus, Bolda noted, is close to being registered in California.
All of the treatments showed significantly higher crop yields in Monterey from April through August compared to the untreated control. The treatment of KPAM plus crop termination then Tri Clor 80 had the highest yields.
Vine Mealybugs in California Vineyards Continue to Challenge Control Efforts

New insecticides, new combinations of insecticides, biological controls and improved mating disruption to control vine mealybug (VMB) are all under study, but costs and necessary control levels are issues, said UCCE Assistant Specialist Kent Daane.
The invasive vine mealybug is the most problematic of all mealybug species and causes the most damage in California vineyards. Damage by the vine mealybug is similar to that of other grape-infesting mealybugs in that it produces honeydew that drops onto the bunches and other vine parts and serves as a substrate for black sooty mold. Mealybugs also will infest grape clusters. Vine mealybug can vector grapevine leafroll associated viruses which have been associated with sudden vine death.
During dormancy and early spring, VMB are found primarily on the trunk, canes and roots, but this can vary by vineyard location. In the spring, VMB moves onto canes and later onto leaves. In the summer, VMB moves into grape clusters. The trunk and the bark of canes provide refuge from insecticides, including systemics and natural enemies.
Ants can be a sign of a VMB infestation. Daane said that ants give refuge to VMB and also improve their habitat by removing honeydew. This tending by ants, increases VMB populations.
Bark wetness is a sign of a mealybug infestation as honeydew production increases. Leaf drop in June can also be sign of an infestation. If a VMB infestation is suspected, crowns and trunks can be inspected in the spring for adult females and crawlers. Starting at bloom, cordons, canes and basal leaves should be inspected. When fruit is present, clusters can be scouted for signs of VMB. Pheromone traps in vineyards from August to October can give record of VMB numbers and indicate numbers for next season. Daane warned that it is common to have high trap counts with little actual crop damage.
Increasing resistance to chemistries used in the past to control VMB make it important to rotate those chemistries which are still effective. Daane said there are both conventional and OMRI-approved insecticides to control VMB, but none provide 100% control.
“Movento is the best, but we are seeing cracks in control,” he warned.
Use of mating disruption devices in the vineyard can help with control. They work best with low VMB pressure. Daane said the effect is better in the second or third year. Ants also must be controlled.
Input Sought on Pesticide Notification Proposal

The California Department of Pesticide Regulation (CDPR) is in the process of developing a statewide pesticide application notification system. Roger Isom, president of Western Agricultural Processors Association, participated in a DPR focus group session in August and reported on this issue at the 2021 Crop Consultant Conference.
Input from the agriculture industry on this proposal to require notification of a pesticide application is vital, said Isom. He urged all growers and PCAs to voice their concerns about the draft proposal to CDPR. Public webinars on the proposal are being held this month. Isom said the draft regulation would be announced in spring 2022.
Unknown at this time are the questions of who will be notified of a planned pesticide application, when will they be notified, how do they get notified, what products are covered and what application methods are covered.
At this time, it is only a proposal, and details are not decided, but Isom warned that the governor has indicated that notification is a priority for him and $10 million has been directed to develop the proposal.
Isom said this action was triggered by Assembly Bill 617 and an air quality reading in Shafter in Kern County that measured 1,3-D; however, there was no known application within seven miles of the measurement. AB 617, signed by Governor Newsom in 2017, establishes a community-scale emissions abatement program and updates air quality standards for sources that contribute to poor air quality.
Isom pointed out that only three states have some type of notification system for pest control applications: Florida, Maine and Michigan. Monterey County has a program for fumigation application notifications for schools. The public is eligible for notification and it is done five days prior to a fumigation. Currently in Kern County, notification is only for restricted use materials and is only required to growers in surrounding areas of the planned application.
Isom noted that in some instances, anyone is able to sign up for notifications, and in the past, it was found that the majority of those requesting notification did not live in the area of the application. In Florida, those requesting notification must be on adjacent or contiguous property up to a half mile.
Isom said any form of this rule should be statewide, reasonable and justification for those asking to be notified of a pesticide application. Public webinars are being held this month. Written comments can be submitted to CDPR.
An Update on Managing Walnut Mold

UC Davis Plant Pathologist Themis Michailides reported recent research findings on walnut mold and management at the 2021 Crop Consultant Conference.
The main pathogens of walnut mold are Alternaria, Fusarium species and Aspergillus niger. Botryosphaeria and/or Phomopsis canker and the bacterial disease walnut blight are also cause for mold in orchards.
” width=”300″ height=”260″ />
Brown Apical Necrosis is a special type of walnut mold that always starts from the stylar end of the nut and is sometimes associated with the bacterial disease walnut blight. Michailides confirmed that there is a strong correlation of fungi causing walnut mold with the same fungi attacking and decaying the hulls. The incidence of mold starting from the stylar end was significantly greater than that of mold starting from the stem end, suggesting possible stylar infection at bloom. In trials over two years, sprays at two to three weeks before hull split and early hull split reduced walnut mold in both early and late walnut cultivars.

In the U.S. standards for grades of walnuts, mold is listed as damage when attached to the kernel or when white or gray mold affects a portion of the surface of the kernel. Potential for mold development in a walnut crop increases as harvest is delayed. Sunburnt nuts, windfalls on the ground for more than 15 days, nuts infested with navel orangeworm, nuts with large stem openings and larger nuts are also subject to mold development.
Michailides said levels of mold reported in recent years included a Chandler sample from Tulare County showing 21% mold, grade sheets from 2020 from Butte County areas near the Sacramento River with 10% to 16% mold and, in 2019, reports of 10% to 20% mold in Butte and Glenn counties.

In his presentation, Michailides noted that previous studies on walnut mold showed that mid-May to mid-July sprays reduced Botryosphaeria and Phomopsis canker and blight, but they did not reduce molds caused by Alternaria or Fusarium. He said the fact that fungi-causing mold are the same as those that colonize the hulls led the decision to move towards sprays before hull split and early hull split.
To reduce incidence of mold in walnuts, Michailides said applying Merivon at three weeks prior to hull split reduces mold related to Botryosphaeria, Phomopsis and Alternaria. Adding Tebuconizole to the tank mix will increase efficacy against Phomopsis. To further increase efficacy, apply Rhyme at 20% to 30% hull split. If the high level of control is not needed, Rhyme can be applied at 20% to 30% hull split.
California Tree Nut Conference
West Coast Nut magazine is offering California tree nut growers a rare opportunity to network across the industry at the second annual California Tree Nut Conference in Tulare Nov. 3. In addition to the usual continuing education seminars and industry trade show, growers attending this year’s free event will hear from California Agriculture Secretary Karen Ross, who will discuss priorities for the California Department of Food and Agriculture and how the agency will support nut growers in meeting those priorities.
In addition, growers will interact with commodity board leaders for the state’s top nut crops in a leadership panel titled “Where Are We Heading?” Panelists will include Michelle Connelly, executive director and CEO of the California Walnut Board and Commission, Richard Waycott, CEO of the Almond Board of California, Richard Matoian, CEO of American Pistachio Growers, and Mark Hendrixson, director of the California Pecan Growers Association.
“Given the many challenges nut growers have faced over the last year, we are using our conference to address big-
picture issues that impact the bottom line for nut growers in California,” said Jason Scott, Publisher of West Coast Nut magazine. “At the same time, we understand that continuing education and networking opportunities with industry suppliers are also important, and we have plenty of that as well.”
A morning panel on “Irrigation Technology and Automating Monitoring Systems in Tree Nut Crops” will feature UC Davis irrigation experts Ken Shackel and Isaya Kisekka, as well as industry suppliers, growers and consultants. They will discuss existing and emerging technologies to determine soil and plant water status for data-driven irrigation management in nut crops.
After the trade show break and post-harvest nutrition demonstration, CEU talks related to nematode management and new technology for monitoring and managing nut pests will be featured. The California Tree Nut Conference will be held from 7 a.m. to 1 p.m. at the Tulare County Fairgrounds in Tulare. Registration is free and can be done online at WCNGG.com or by calling the JCS Marketing office at (559) 352-4456.
Stinkbugs Can Reduce Marketable Tomato Yields

Monitoring for stinkbugs and managing weeds to eliminate their overwintering habitat are two important pre-plant steps in processing tomato production.
The Pest Management Strategic Plan for California Processing Tomato Production, part of UC’s Statewide IPM Program, notes that stinkbug infestations are capable of reducing marketable tomato yields by up to 40%. The plan was prepared by Tunyalee Martin, Cassandra Swett, Amber Vinchesi-Vahl and Stephanie Parriera.
Stinkbugs cause the most damage in processing tomatoes in the southern growing region. They cause damage from late fruit set through fruit ripening. Consperse stinkbug is listed as the most damaging species, but southern green stinkbug has also been found to damage tomato crops. There is concern that infestations by the invasive brown marmorated stink bug will occur in the future due to this pest’s movement into other California crops.
Stink bug feeding causes calluses and discoloration of the fruit. Feeding on mature fruit can open the way for secondary infections and severe rot.
Thresholds for stink bug numbers are not used in pesticide application decisions due to this pest’s ability to cause damage even at low numbers. Pheromone lure traps in the fields can assist with early detections.
The strategic plan notes that weed management is an important component in control of stinkbugs. Removing weeds that are attractive to stink bugs, including little mallow, Russian thistle and mustards, is recommended.
Insecticides are used for management, but do not always provide a high level of control. UCCE trials conducted in Fresno County suggest that control is best when a neonicitinoid and pyrethroid are tank-mixed.
Coverage is critical but difficult as stink bugs can be at or below the soil surface during part of the day. Applications are reported to be more successful with an air-assisted sprayer.
In addition to pre-plant monitoring, the strategic plan recommends insecticide applications from planting to pre-bloom if stinkbugs are detected in the field. From bloom to early fruit set, monitoring with pheromone traps or beat trays will allow for early detection of infestations. An insecticide application is recommended if stink bugs are detected in the field.
Monitoring should continue from late fruit set to first red fruit.
Harvesting as soon as possible is a good strategy to avoid additional stinkbug damage and rot from yeasts, but is not always possible due to other factors, including predetermined arrangements with processors.
Verticillium Wilt, Freeze Damage Looks Similar in Oil Olives

Super-high-density oil olive plantings have increased in acreage in several growing regions in California while table olive acreage continues to decline. In San Joaquin County, nearly 5,500 oil olive acres are now in production. In their Field Notes publication, UCCE Advisor Mohammad Nouri and UC Kearney Plant Pathologist Florent Trouillas described the challenges of diagnosing symptoms with different causes.
Symptoms of the fungal disease Verticillium wilt and freeze or frost damage can be similar, Nouri noted.
The soilborne fungus that causes Verticillium wilt has both non-defoliating and defoliating types. Symptoms usually begin in the spring and slowly worsen in the summer. The non-defoliating type can cause rapid dieback and wilting, especially in young trees as the pathogen invades the tree’s vascular system. The defoliating type causes early drop of asymptomatic green leaves from individual twigs and branches.
Look inside the affected branches to tell the difference. With Verticillium wilt, compared to freeze or frost damage, obstructed vessels become dark and inner vascular tissues show dark streaking. Advanced stages include shoot wilting, dieback and foliar necrosis, which are also associated with freeze damage.
Nouri said olive trees affected by cold temperatures during the growing season have frost damage. Freeze damage occurs in late fall or winter while trees are dormant. Initial damage includes tip dieback, lack of luster to the leaves and curling up of leaves as well as some necrotic or chlorotic lesions and leaf drop.
His observations are that a dry fall may make freeze damage worse. Cutting back on irrigation in September and avoiding nitrogen applications can help slow growth and may help trees harden off before a sudden freeze event. If a freeze is forecast, having a moist soil surface can help store more heat. Symptoms of freeze damage will appear when the weather warms. Sufficient moisture helps trees recover. Pruning should be delayed until warmer weather begins to stress the trees to see where recovery is possible as new shoots grow. Branches and limbs damaged by freeze will be easily identified and can be removed. The soil profile should be wet to the depth of rooting when spring growth begins to stimulate root activity.
Nouri said that trees severely affected by freeze damage will have less total growth, which will reduce the nitrogen requirement, but will stimulate vigorous re-growth similar to heavy pruning. Fertilizer decisions for the growing season following a freeze should be based on current soil reports and leaf analysis.
Monitor Rust Levels in Almonds
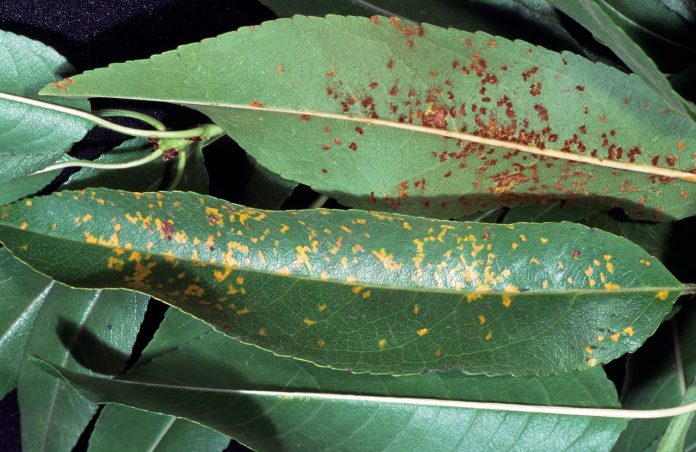
A sporadic disease in California almond growing regions, rust is linked to humid growing conditions and excessive levels of nitrogen.
Vigorous, higher-density plantings and microsprinkler irrigation with longer, more frequent irrigations can contribute to higher humidity and more accumulated leaf wetness hours resulting in more disease.
UC’s IPM Almond Pest Management Guidelines report that almond trees can be defoliated very rapidly when a rust infection becomes severe. Early defoliation deprives trees of needed nutrients and can reduce the following year’s bloom if not controlled. Rust can be a problem in non-bearing orchards where fungicides have not been applied.
This fungal disease survives from one season to the next in infected plant material.It is important to know the levels in the current and previous seasons as indicators for risk. This helps determine at what level the inoculum may or may not be present and disease progress can be monitored.
Rust appears as small yellow spots on the upper surface of leaves. On the lower surface of the leaf, spots take on a rusty red appearance when the rust-colored spores produced in the lesions erupt through the surface. These spores are spread by air movement and infect other leaves to continue the disease cycle. Young twigs may be infected, but twig lesions are not common.
In almond orchards with a history of rust, the guidelines recommend applying sulfur five weeks after petal fall and follow four to five weeks later with a quinone outside inhibitor (FRAC group number 11).
Two or three applications may be needed in orchards that have severe rust infections. To be effective, the fungicide application must be made before rust symptoms appear.
Sac Valley Orchard News recommends considering a foliar zinc sulfate fertilizer spray to get zinc into the trees as the leaves start to naturally drop. This will also hasten leaf fall and reduce infected leaf carry-over into next season. The application should not be done until late October or early November to allow leaves time to continue making photosynthate and build up energy storage in the trees after harvest.
Unchecked, the inoculum may build up, overwinter on the trees and infect leaves the following spring. In southern growing regions, leaves of some cultivars, such as Sonora, may remain attached over the winter and provide inoculum for new infections as leaves emerge the following spring.
Fungicides effective on rust can be found at the UC IPM guidelines or the Efficacy and Timing of Fungicides Publication.
Sulfur as Part of a Powdery Mildew Control Program

Sulfur applications in winegrape vineyards can kill powdery mildew spores that have not yet caused infection, kill incoming spores and cut down newly established colonies.
Sulfur has a long history of controlling powdery mildew, said UCCE Viticulture Advisor Larry Bettiga. In a UC Ag Expert webinar, Bettiga said sulfur was first used in 1850 to control powdery mildew. More sulfur is used in vineyards than any other major type of pesticide.
Powdery mildew has two spore types. Chasmothecia spores overwinter and cause initial spring infections. Conidia spores cause growing season infections. Powdery mildew begins on grapevine leaves as chlorotic spots on the upper leaf surface. Signs of the pathogen appear a short time later as white, webby mycelium on the lower leaf surface. As spores are produced, the infected areas have a white, powdery or dusty appearance. On fruit or rachises, the pathogen may colonize the entire berry surface.
Benefits of sulfur use in vineyards include good efficacy for powdery mildew control. There is low potential for resistance development by the pathogen. Compared to fungicides, sulfur applications cost less and they are acceptable in organic and biological production systems. Sulfur also helps with suppression of other pest populations.
Negatives of sulfur applications include increased potential for hydrogen sulfide production, drift from dust application and the potential for plant tissue toxicity, and it can be detrimental to some natural enemies. The potential for sulfite production during fermentation can be avoided if sulfur treatments end in the vineyard five weeks prior to harvest.
Sulfur applications are also less costly than synthetic fungicide applications. At a rate of 15 pounds per acre and adding the cost per acre for application, Bettiga said the total was less than $20 per acre. This was compared to Rally, Quintec and Pristine at $51.60, $53.92 and $74.60 per acre, respectively.
In an evaluation of sulfur and other fungicide tank mixes, after six applications, sulfur treatments alone resulted in 2.8 affected berries per cluster while tank mixes scored one or less.
Efficacy is influenced by the mode of action phase. As a contact, sulfur is not influenced by temperature. As a vapor, activity below 59 degrees F is very limited.
Density of foliage and canopy management will also affect efficacy of sulfur and other fungicides. Sprayer settings, including travel speed, volume and velocity of air, nozzle selection and droplet size, and nozzle orientation to canopy play a part in an effective application.


























