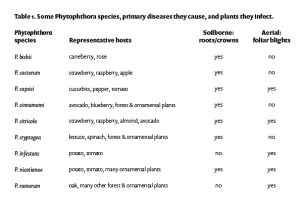
The goal of airblast spraying is a uniform pesticide deposition of a known, prescribed pesticide rate throughout the entire target (tree canopy). Done right the first time, a good spray job saves the time and money of a second spray plus income lost due to crop damage in the case of a poor first spray (in tough economic times, a second spray for the same problem may not be in the budget.)
There are several steps to achieving this goal. Skipping any step will reduce spray efficacy and efficiency.
Step 1: The sprayer should travel at an appropriate speed to allow spray to reach the treetops. Too slow sprayer speed wastes time, too fast means poor coverage in the treetops and the risk of income loss due to crop damage.
Step 2: Point larger nozzles at thicker canopy (more leaves and nuts). For most orchard crops, this means 65% to 80% of the spray flow (gallons per minute) should be applied through the top half of open nozzles.
Step 3: Measure gallons per acre sprayed and, using total spray tank volume, determine the amount of pesticide product to add to each tank, to match your PCA’s recommendation.
Step 4: Check coverage with water-sensitive paper (WSP) placed in the canopy.
Details
Ground speed
Airblast spraying uses air from the sprayer’s fan(s) to move the pesticide throughout the tree. If the fan’s air doesn’t reach the treetops, the pesticide won’t either. Ground speed is a simple and effective way to adjust air movement through the canopy, especially between bloom and harvest when spray coverage is most challenging.
The sprayer should travel fast enough so air from the sprayer’s fan reaches up through the tree to just above the tops of the tallest trees. To check this, at a time of day with little to no wind, tie a short (18-inch) length of surveyor’s ribbon to a section of PVC pipe or conduit and run the tubing up through the middle of a tree to a height just above the tallest trees in a planting. With the sprayer fan “on,” drive the sprayer past the tree with the flagging at tractor and sprayer settings you think is appropriate (e.g., full sprayer air delivery and 2.25 MPH sprayer speed).

If the flagging flutters out to 45 degrees from the vertical as the sprayer passes the tree, the speed is appropriate for that planting at that time of the season. If the flagging just barely moves or doesn’t move at all, repeat the process with slower tractor speed. If the flagging kicks up to the vertical (180 degrees from dead hang), repeat the process at a faster tractor speed. Record the tractor and sprayer settings that deliver air movement from the sprayer fan to just above the canopy. Calculate the acres per minute sprayed at that ground speed by multiplying ground speed (feet per minute) by the row width. Note: If spraying on a day with slight winds, drive slower, delivering more fan air to compete with the wind and better cover the upper canopy.
Nozzle selection
With a gallons per acre (GPA) target from your PCA and the appropriate sprayer speed measured with the aforementioned “flagging on a pole” process, calculate the sprayer output (gallons sprayed per minute; GPM) needed.
Gallons per minute = (Gallons per acre) x (Acres per minute)
Now select nozzles to deliver the GPM you just calculated (on paper). More spray should be applied to areas of the tree with more leaf area. Upper-canopy locations often hold more crop than the rest of the tree and are the toughest to cover. Extra spray volume with larger nozzle size targeted there will deliver more uniform coverage.
Step 1: Park the sprayer in the orchard and look where the different nozzle ports are located. Tying flagging to the nozzle ports and running the fan can help show you which ports point where in the tree.
Step 2: Using the manufacturer’s catalog and desired system pressure (for example, 150 psi), select nozzle sizes to locate on different nozzle ports. The goal for mature trees is 65% to 80% of the total GPM going out the top half of the open nozzles. That is, if there are 16 nozzles per side of a sprayer that should be open in a particular orchard based on the sprayer and tree size, the top 8 should have most of the total GPM. Using the same nozzle size at every nozzle port will, at best, overspray the lower canopy while delivering good/decent coverage to the treetops (as long as the ground speed is right.)
Gallons per acre
With the ground speed and nozzles selected, determine the GPA by checking the math you just did in the previous step. Park the sprayer on flat ground and completely fill the tank with clean water. With the nozzles just selected on the sprayer and using the sprayer and tractor settings for the right/appropriate ground speed, turn on the spray booms for a measured amount of time (one minute, two minutes, etc.) and then shut off the flow. Refill the sprayer with clean water using calibrated buckets or a hose with a flow meter to measure how much water was sprayed in the time the nozzles were “on.” Calculate GPM from the volume sprayed and the run time. Adjust GPM, as needed, using the system pressure or by changing nozzle sizes or parts (e.g., two- or four-hole swirl plates for disc/core nozzles) to deliver the recommended GPA.
Check coverage
Water-sensitive papers (WSP) are small cards with yellow coating on one side that turn blue where water (or fingerprints) touches the surface. To check spray coverage, place WSP at different heights in the trees in the orchard. This can be done several ways. If you have a pruning tower, use it to get up into one or more trees in the orchard and directly clip WSP to leaves or attach to nuts. Flag each WSP location so you can find it later. Another method is to attach WSP at different heights on a PVC pole and run the pole up through the middle of the tree canopy.
Once WSP are up in the canopy, spray clean water down the row where WSP are placed using the tractor settings and nozzle selection/location determined earlier. Take down WSP after and compare upper- and lower-canopy locations to see if coverage is generally uniform. You can measure coverage with a smartphone camera and apps, but a visual scan should be enough. Are the lower cards all blue? If so, the lower canopy is getting too much spray. One possible fix for this is to change out lower nozzles for a size smaller and repeat the test. If the upper cards are not getting much coverage, increase selective nozzle sizes that target the upper canopy and/or slow down the sprayer.
Spraying when relative humidity is low (<40%) can cut spray deposition in the upper canopy in half compared to spraying when relative humidity is higher (early morning). This can lead to poor pest control and/or development of pesticide resistance. Especially in warm summer months with low daytime humidity, night and early morning spraying is important to achieving good spray coverage.
Effective pest control with pesticide(s) is a key backstop in a good, cost-effective IPM program. Good spray coverage (and material selection/spray timing) ensures the backstop is solid.