Phosphorus (P) availability limited food production and human population until the Green Revolution. After the second World War, mineral fertilizers and powerful new pesticides drove record yields and exponential world population growth. While nitrogen fertilizer usually takes all the credit, phosphorus is a close second, and even the major limiting element under some conditions. Mining P-rich ore introduced more P to the biosphere than ever before. Instead of relying on biological P cycling, mineral fertilizer now keeps our fields productive through years of back-to-back planting. P fertilizer provides undeniable improvements to yield and crop quality, but leaks in the system destabilize surrounding ecology, causing a cascade of effects that shift global P cycling. With fertilizer prices on the rise and environmental impact mounting, everyone can benefit from improving P management.
All living organisms require P. It constitutes about 9% of our DNA, it is the backbone of the phospholipid fatty acids giving our cells their structure, and P is a critical component of Adenosine Triphosphate (ATP), the powerhouse of the cell. Plants take up most of their P via the roots as the anion phosphate (P2O43-). Crops require lots of P early in development to support rapidly growing cells. P is required at every stage of growth, first to support DNA transcription and translation, then to build the cellular structure and to supply energy needed to carry out all the activity. Ensuring early access to enough plant-available P drives vigorous root growth and sets the crop up for success.
Activity and Availability
P bioavailability limits many natural ecosystems, and plants have evolved several ways to gain access to the essential element. P is immobile in soil, and most of it is tied up in mineral pools with very little available as phosphate in soil solution. Some P minerals are very stable and resist dissolution. Others readily dissolve with slight adjustments in pH or enzyme activity. Plants and fungi take advantage of the labile mineral pool by excreting phosphatase and lowering the pH in their direct vicinity. Mycorrhizal fungi bond with plant roots, extending their hyphae far past where the plant can reach to bring back phosphorus and water in exchange for photosynthate. Soil organic matter also stores P, and microbial activity releases phosphate into solution according to population dynamics and access to carbon and nutrients.
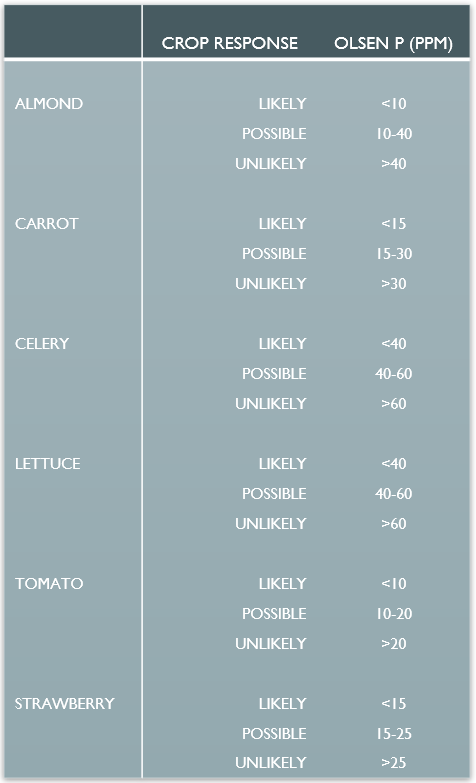
Most P fertilizer recommendations are based on observed crop response to fertilization at different soil P concentrations. Many studies in the western region show that crop yield and quality increases when fertilizer is applied to soil with less than 40 ppm P measured by the Olsen test. Soil containing 40 ppm P holds roughly 180 lbs plant available P2O5 in the upper six inches. Celery takes up about 100 lbs of P2O5 per acre, and about 70% of the P is removed from the field with the harvested crop. Soils with 100 ppm P have 460 lbs P2O5 per acre down to six inches, providing more than five times the P demand of most vegetable crops. Most crops send roots below six inches, gaining access to even more P.
Phosphorus fertilizer gives crops immediate access to P, circumventing the slower biological cycling. Phosphoric acid, monoammonium phosphate and other sources initially spike soil solution phosphate, but the effect does not last. In calcareous and high-pH soils, P eventually disappears from the plant available pool as it binds with calcium to form the mineral apatite. Under acidic conditions, phosphate precipitates with iron and aluminum hydroxides. The P fixation rate depends on many factors, including pH, temperature, moisture and the concentration of other compounds in soil solution. Growers apply more P fertilizer every year to meet immediate crop needs, even though total soil P levels continue rising.
Environmental Impacts
While adsorbed P might not be accessible to the crop, the extra nutrition disproportionately impacts freshwater and marine environments when it escapes the farm via runoff or wind erosion. Relatively small increases in P concentration in lakes, streams and ocean water cause major ecological shifts. High N and P levels induce eutrophication by triggering algal blooms that block sunlight from penetrating the water’s surface layer. Unable to photosynthesize, aquatic plants die and sink to the bottom, introducing an overabundant food supply to microorganisms. Aerobic metabolism depletes the water’s oxygen concentration as microbes decompose the plant material. Oxygen diffusion down to lower depths can’t keep pace with the consumption rate. Hypoxic zones drive away or kill off fish, and the aquatic ecosystem unravels, leading to permanent dead zones under the worst conditions.
Researchers point to organic matter as the solution to almost every soil quality challenge, and phosphorus is no exception. Increasing soil organic matter and microbial activity helps prevent erosion and increases the bioavailability of P already in the soil. Microbial metabolism releases carbon dioxide, dissolving calcium phosphate minerals. Enzymes and organic acids also liberate phosphate, while mycorrhizal networks mine phosphorus from parts of the soil profile that plant roots cannot reach. Meanwhile, microbial activity and organic matter build soil structure, forming stable aggregates that resist erosion from water and wind. One major windstorm can blow away an inch of topsoil carrying away valuable phosphate fertilizer. Soil with 100 ppm P concentration holds almost 80 pounds of plant-available phosphate in the upper inch of soil. Many fields have accumulated P to well over 200 ppm, doubling or tripling the cost of eroded P.
Phosphorus fertilizer is a valuable tool and a mainstay of almost every fertilizer regimen. Increased yields and reasonably priced fertilizer keep growers applying mineral P every season. While a heavy P application may have significantly increased yield the first couple of years, continued applications at the same rate may have little effect. Soil tests can help determine baseline P content and the soil’s adsorption capacity. Water quality, soil pH and calcium content affect how quickly P fertilizer precipitates out of the plant-available pool. Management practices like splitting P into several applications or applying it with an organic amendment can help keep a steady supply of P in plant-available form. Like most agricultural challenges, the best solutions are multipronged, with many little adjustments adding up to dramatically improve the big picture. Better P management will protect our freshwater and marine environments, enhance crop quality and even save growers some money.
References
Brady, Nile C. and Weil, Ray R. (2008). The Nature and Properties of Soils. Fourteenth Edition. Pearson Prentice Hall.
Filippelli, Gabriel. (2008). The Global Phosphorus Cycle: Past, Present, and Future. Elements. 4. 89-95. 10.2113/GSELEMENTS.4.2.89.
Geisseler, Daniel. (2015). California Fertilization Guidelines. Fertilizer Research and Education Program. http://geisseler.ucdavis.edu/Guidelines/Home.html
Liu, Guodong, Li, yuncong, & Gazula, Aparna. (2019). Conversion of Parts Per Million on Soil Test Reports to Pounds Per Acre. University of Florida Extension. https://edis.ifas.ufl.edu/publication/hs1229
Wyant, Karl A., Corman, Jessica R., & Elser, James J. (Eds.). (2013). Phosphorus, Food, and our Future. Oxford University Press.