Ultraviolet-C light (UV-C) for crop protection is not a new concept, but how we integrate the technology into pest and disease management programs is. Field application of UV-C has successfully reduced powdery mildews of strawberry (Onofre et al. 2021), cantaloupe (Lopes et al. 2023) and grapevine (Ledermann et al. 2021, Gadoury et al. 2023). This was possible due to key findings that enhanced efficacy by applying UV-C during a dark period that continued for at least four hours after application (Janisiewicz et al. 2016a, Suthaparan et al. 2016a, Onofre et al. 2021). This dark period allows the UV-C damage to be permanent by bypassing the robust fungal photolyase repair mechanism that is driven by the blue and UV-A components of sunlight (Beggs 2002). Applying UV-C followed by a dark period (i.e., a lower, non-phytotoxic dose) can be used to suppress pathogens effectively (Suthaparan et al. 2014, 2016b, Janisiewicz et al. 2016b, Onofre et al. 2021). Pioneers in UV-C application for crop protection (David Gadoury with Cornell University and USDA scientists Fumiomi Takeda and Wojciech Janisiewicz) have previously written articles in Progressive Crop Consultant. These articles further describe past UV-C research that has led to the successful application of UV-C.
Our research conducted at Washington State University, led by Michelle Moyer, was in collaboration with Gadoury and Walt Mahaffee with USDA to expand UV-C applications for field-scale pest and disease management in grapevines. Our goal was to expand our knowledge of how UV-C can be used as a non-pesticidal alternative to suppress grapevine powdery mildew (Fig. 1) in Eastern Washington State Vitis vinifera vineyards through testing different timing and intervals for UV-C application. We additionally explored UV-C effects on basic fruit chemistry. The results of our research (McDaniel et al. 2024) are now published open access at American Journal for Viticulture and Enology.

Experiment Methods
Our field-scale UV-C (Fig. 2) array was based on designs from Gadoury and Mahaffee. It was built as an over-the-row triangular arch with 12 ballasts to power 24 UV-C lamps backed by polished aluminum reflectors. It was supported by a metal tower attached to the three-point hitch system of a tractor. Thick PVC strips were mounted on each end of the array like curtains to contain UV-C within the apparatus. The framework was built by VineTech Equipment in Prosser, Wash. A dose of 200 J/m2 was achieved by adjusting ground speed based upon the array length and mean irradiance at the approximate height of the fruiting zone in the vineyard (Gadoury et al. 2023). UV-C dose is based on how long the vine is exposed; a longer array, slower speed or more bulbs would equal a higher dosage. UV-C treatments were applied 30 minutes post-sunset to allow for an optimal dark period.
Our trials conducted at the WSU research vineyard in Prosser tested UV-C intervals of weekly or twice-weekly in two different timing strategies to manage grapevine powdery mildew from 2020-22. Early season timing trial consisted of UV-C treatments made from early shoot growth to pre-bloom followed by a fungicide spray program post-bloom. Season-long timing trial had UV-C treatments that replaced all fungicides from early shoot growth to three weeks post-fruit set. Early season UV-C treatments were compared against three controls and season-long UV-C treatments were compared against two controls.

Treatments for the early season trial:
1) Early weekly UV-C
2) Early twice-weekly UV-C
3) Early unsprayed, no fungicide treatments from early shoot growth till pre-bloom
4) Unsprayed, no fungicide treatments for the season
5) Fungicide program, based off typical spray programs in Eastern Washington Vineyards
Treatments for the Season-long Trial:
1) Weekly UV-C
2) Twice-weekly UV-C
3) Unsprayed, no fungicide treatments for the season
4) Fungicide program, based off typical spray programs in Eastern Washington Vineyards
Grapevine powdery mildew was visually rated as a disease severity percentage on leaves and clusters from bloom and continued until harvest. Severity ratings were converted into an area under disease progress curve (AUDPC), which quantifies disease intensity over time, providing an understanding of how disease accumulates throughout the season. The following berry harvest metrics were measured: yield, soluble solids, titratable acidity and pH. Berry skin tannin and phenolic concentrations were additionally measured from season-long treatments following the Adam-Harbertson Methods (Harbertson et al. 2002, 2003) as phenolics are shown to increase with sunlight exposure.
Results
The hot and dry weather conditions in Eastern Washington State for 2020 and 2021 did not favor grapevine powdery mildew infection. In 2021, the daytime high temperatures from bloom to veraison exceeded 35 degrees C for 17 consecutive days. The 2022 vintage was dramatically different than the previous years, creating a favorable climate for grapevine powdery mildew infection with below-average temperatures and above-average precipitation. The low disease pressure experienced in 2020 and 2021 influenced our ability to evaluate the potential reduction of disease. It is hard to separate disease ratings when low to no disease is present.
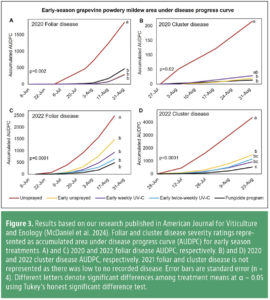
Early season UV-C treatments significantly reduced foliar and cluster disease severity relative to the unsprayed control in 2020 and 2022 (Fig. 3). In 2021, due to extended high temperatures throughout the growing season, there was low foliar and no cluster disease in the vineyard, resulting in no separation of UV-C treatments, the fungicide program and untreated vines. Overall, early season UV-C, either weekly or twice-weekly, was as effective as the fungicide program for reducing disease. This indicates UV-C could replace standard fungicides early season in a grapevine powdery mildew management program for Eastern Washington.
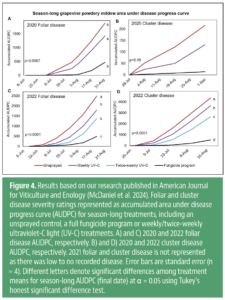
Season-long UV-C treatments (Fig. 4) in 2020 did not significantly reduce foliar or cluster AUDPC relative to the unsprayed treatments. In 2021, due to high temperatures and low precipitation, there were no differences between any treatments in foliar AUDPC, and there was no observable disease on clusters regardless of treatment. In 2022, weekly and twice-weekly UV-C applications significantly reduced foliar AUDPC relative to the season-long unsprayed control. Cluster AUDPC in 2022 was significantly reduced with weekly and twice-weekly UV-C applications with twice-weekly UV-C performing the best. In 2022, both UV-C treatments did not significantly reduce foliar, or cluster disease compared to the fungicide program. This leads us to believe that improvements to UV-C application treatments can be improved. Season-long UV-C did not affect the yield, pH, titratable acidity or Brix of the grapes. The effects of season-long UV-C on tannins and phenolics were inconsistent year-to-year, suggesting factors other than UV-C were more influential on these measures. Nonetheless, season-long or exclusive UV-C for grapevine powdery mildew management requires further evaluation under Washington State conditions.
While not always statically significant, the consistent trend in reduction in powdery mildew disease as a result of weekly or twice-weekly UV-C treatment indicates the incorporation of UV-C into a vineyard IPM program could be an effective alternative for powdery mildew management without compromising fruit quality. To improve UV-C efficacy we believe that canopy management could play a critical role. For this experiment, we did not implement any canopy management practices as we were trying to induce grapevine powdery mildew disease. As with many foliar pesticide applications, coverage of the desired target is required for efficacy. A dense and complex canopy architecture (which our canopies were in this experiment) makes access to the fruiting zone a challenge. This might explain the more-consistent disease suppression when UV-C was applied only in the early season as canopies were less dense and allowed greater UV-C penetration. This also suggests UV-C efficacy may respond favorably to pruning and training systems that open canopies and expose the fruiting zone. To allow light (or spray) penetration to the fruiting zone, the following practices are important: shoot training, shoot thinning and leaf removal.
Future Studies
Though we are pleased with these results, considering the benefits and potential negative impacts must be addressed for grower adoption. One main benefit of UV-C is it can be used in rain or wind, making it less environmentally dependent compared to typical pesticide sprays. Another plus, there is immediate reentry after treatment. This technology provides a residue free option, uses no water, and can be used when fungicide resistance is present in populations. All these examples make UV-C a potential tool for a sustainability forward pest management program. The cons to this technology are application is labor-intensive and requires nighttime applications. Management of grapevine powdery mildew with UV-C would demand at least weekly applications, most likely twice-weekly for improved efficacy, which could be a substantial amount of tractor hours. As it is a new tool, it requires either you build it yourself or wait for commercial units. Lastly, the effects to beneficial insects are still unknown. Studies that explore these challenges will be important for grower adoption.
UV-C research is still being continued at WSU within the Moyer lab by graduate student Jesse Stevens based on the results from these findings. They are exploring how canopy management practices, such as shoot thinning and fruit zone leaf removal, can increase the efficacy of UV-C applications for managing grapevine powdery mildew.
Alexa McDaniel is now a Viticulture Extension and Research Scholar at North Carolina State University where her program aims to provide extension education materials for best viticulture practices and field-applied research focuses on pest and disease management. Additional information on UV-C can be requested from McDaniel (almcdan2@ncsu.edu) or Michelle Moyer (michelle.moyer@wsu.edu).
This work was funded by the Washington State Grape and Wine Research Program. The author would like to thank Bernadette Gagnier, Jake Shrader, Maria Mireles, Charlotte Oliver and Margaret McCoy for their help in this project.
References
Beggs CB. 2002. A quantitative method for evaluating the photo-reactivation of ultraviolet damaged microorganisms. Photochem Photobiol Sci 1:431-437. DOI: 10.1039/B202801H
Harbertson JF, Kennedy JA and Adams DO. 2002. Tannin in skins and seeds of Cabernet Sauvignon, Syrah, and Pinot noir berries during ripening. Am J Enol Vitic 53:54-59. DOI: 10.5344/ajev.2002.53.1.54
Harbertson JF, Picciotto EA and Adams DO. 2003. Measurement of polymeric pigments in grape berry extract sand wines using a protein precipitation assay combined with bisulfite bleaching. Am J Enol Vitic 54:301-306. DOI: 10.5344/ajev.2003.54.4.301
Gadoury DM, Sapkota S, Cadle-Davidson L, Underhill A, McCann T, Gold KM et al. 2023. Effects of nighttime applications of germicidal ultraviolet light upon powdery mildew (Erysiphe necator), downy mildew (Plasmopara viticola), and sour rot of grapevine. Plant Dis 107:1452-1462. DOI: 10.1094/PDIS-04-22-0984-RE
Janisiewicz WJ, Takeda F, Nichols B, Glenn DM, Jurick II WM and Camp MJ. 2016a. Use of low-dose UV-C irradiation to control powdery mildew caused by Podosphaera aphanis on strawberry plants. Can J Plant Pathol 38:430-439. DOI: 10.1080/07060661.2016.1263807
Janisiewicz WJ, Takeda F, Glenn DM, Camp MJ and Jurick II WM. 2016b. Dark period following UV-C treatment enhances killing of Botrytis cinerea conidia and controls gray mold of strawberries. Phytopathology 106:386-394. DOI: 10.1094/PHYTO-09-15-0240-R
Ledermann L, Daouda S, Gouttesoulard C, Aarrouf J and Urban L. 2021. Flashes of UV-C light stimulate defenses of Vitis vinifera L. ‘Chardonnay’ against Erysiphe necator in greenhouse and vineyard conditions. Plant Dis 105:2106-2113. DOI: 10.1094/PDIS-10-20-2229-RE
Lopes UP, Alonzo G, Onofre RB, Melo PP, Vallad GE, Gadoury DM et al. 2023. Effective management of powdery mildew in cantaloupe plants using nighttime applications of UV light. Plant Dis 107:2483-2489. DOI: 10.1094/PDIS-08-22-1941-RE
McDaniel AL, Mireles M, Gadoury D, Collins T and Moyer MM. 2024. Effects of ultraviolet-C light on grapevine powdery mildew and fruit quality in Vitis vinifera Chardonnay. Am J Enol Vitic 75:0750014. DOI: 10.5344/ajev.2024.23071
Onofre RB, Gadoury DM, Stensvand A, Bierman A, Rea M and Peres NA. 2021. Use of ultraviolet light to suppress powdery mildew in strawberry fruit production fields. Plant Dis 105:2402-2409. DOI: 10.1094/PDIS-04-20-0781-RE
Suthaparan A, Stensvand A, Solhaug KA, Torre S, Telfer KH, Ruud AK et al. 2014. Suppression of cucumber powdery mildew (Podosphaera xanthii) by supplemental UV-B radiation in greenhouses can be augmented or reduced by background radiation quality. Plant Dis 98:1349-1357. DOI: 10.1094/PDIS-03-13-0222-RE
Suthaparan A, Solhaug KA, Stensvand A and Gislerød HR. 2016a. Determination of U V action spectra affecting the infection process of Oidium neolycopersici, the cause of tomato powdery mildew. J Photochem Photobiol B 156:41-49. DOI: 10.1016/j.jphotobiol.2016.01.009
Suthaparan A, Solhaug KA, Bjugstad N, Gislerød HR, Gadoury DM and Stensvand A. 2016b. Suppression of powdery mildews by UV-B: Application frequency and timing, dose, reflectance, and automation. Plant Dis 100:1643-1650. DOI: 10.1094/PDIS-12-15-1440-RE