Applying fertilizers or plant nutrients to foliage has a long history, and there is an extensive number of papers published on the subject. However, it may surprise some to learn that we still do not fully understand how nutrients applied to the foliage cross the leaf cuticle and enter the cytoplasm of plant cells where they can be used in metabolism. The cuticle is the main barrier to absorption of foliar applied nutrients.
Many Factors at Play
All aerial plant parts, including leaves and berries, are covered by a complex structure (a little like our own skin) known as the cuticle. The plant cuticle limits or regulates the transport of water and other substances between the plant and the environment, in addition to protecting plant cells from UV light and discouraging or sensing pests or pathogens. The cuticle is a hydrophobic layer formed just outside the cellulose cell wall that is composed mainly of the cutin polymer (a polyester) with waxes both embedded in it and covering its surface. However, numerous other chemical constituents beyond the scope of this discussion are also present in the cuticle and alter its properties with respect to the transport of any compound across this barrier. While there is still much debate about how different compounds (including plant nutrients) cross the cuticle, two major pathways of transport are known: the non-polar pathway or waxy pathway, and the polar pathway or aqueous pathway.
Compounds that are soluble in oil or an organic solvent, such as some herbicides or even smoke-related phenols, cross the cuticle easily through the waxy pathway because they are basically soluble in it. However, most plant nutrients are polar compounds or ions that are water-soluble, and transport of these substances relies on the aqueous pathway. This aqueous pathway exists because of ‘aqueous pores’ that occur within the waxy cuticle. While these pores are known to exist based on indirect evidence, they have yet to be seen. Aqueous pores in the cuticle become more open or connected as the relative humidity in the air increases. Therefore, transport of foliar-applied nutrient ions is much greater at higher humidity, and spraying foliar nutrients early in the day when humidity is still high is more effective than at midday or in the afternoon when humidity drops. Higher humidity also translates to a longer time that the spray droplets remain wet, thus keeping ions in solution longer, which also facilitates greater transport across the cuticle. Humidity also affects what is known as the point of deliquescence (POD) for different ions, which is the level of humidity needed for a given ion to attract water vapor and remain in solution on a given surface. POD varies for different nutrients, but higher humidity levels increase the chance that POD will be exceeded.
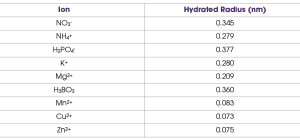
Another property of nutrients or ions related to aqueous pores and transport through them is known as the hydrated ion radius, which is the molecular size of the given ion and those water molecules tightly adhered to it when dissolved in water (Table 1). Many macronutrient ions have a larger hydrated radius when compared to micronutrient ions. A smaller hydrated radius allows for greater transport through aqueous pores estimated from various studies to have a diameter of about 0.3 to 2.0 nanometers. This is one reason that foliar application of micronutrients is more effective than foliar application of macronutrients. However, one must also consider that micronutrients are needed in much, much lower quantities to be in a healthy range. Indeed, it would be difficult, if not impossible, to supply enough nutrients for most macronutrients like N through the foliage alone.
Table 1 shows that the hydrated radius for boric acid is actually on par with the macronutrients. Yet, we know that boric acid and other sources of boron (e.g., Solubor) are used very effectively to alleviate B deficiency in grapevines. This is because uncharged molecules cross the cuticle far easier than charged ions. While it is still not entirely clear, some foliar nutrition experts think that relatively small molecules that are uncharged, such as boric acid and urea, cross the cuticle via the waxy pathway. Indeed, water molecules appear to be transported to some extent via the waxy pathway, allowing some plants to take up significant water directly through their leaves.
Cuticles on the upper surface of leaves (known as the adaxial surface) are generally thicker than the corresponding cuticle on the leaf bottom surface (abaxial surface). When nutrients were applied separately to upper versus lower leaf surfaces, more transport occurred across the bottom side of the leaf, up to four times more. Another practical consideration to increase nutrient absorption is to ensure that sprays are well deposited on the underside of leaves by having good airflow during spraying. This could mean increasing fan speed.
Another consideration important for nutrient transfer is the contact angle of the spray droplets that land on plant surfaces. This property is mostly dictated by cuticle properties, particularly the waxes on the outer surface, and by the physio-chemical properties of the spray solution. Different plant species, different tissues (leaves vs fruits) and even different ages of the tissues along with the prevailing environmental conditions affect cuticle properties that alter contact angles. Spray adjuvants or surfactants can also reduce the contact angle (i.e., help spread out the droplets) and increase transport across the cuticle. Numerous studies using isolated cuticles have shown the importance of spray adjuvants to increase foliar absorption of nutrients. In addition, adjuvants can delay the drying time of droplets and keep the nutrient in solution longer, allowing for greater uptake. It should be noted that there are also cases where the naked nutrients alone (without spray adjuvants) were absorbed just as effectively as when applied with an adjuvant.
What about chelates? This area gets a lot of marketing attention, but due to the proprietary nature of many chelates sold, it is often difficult to untangle advertising from evidence that specific chelates actually improve foliar uptake. While there are cases where chelates have proven more effective than nutrient ions alone, there are also cases where they have not.
Finally, another factor still unresolved regarding foliar uptake of nutrients is the role of stomates and other structures on the external surface of leaves, such as trichomes (leaf hairs) and specialized cells above veins.
Recent findings show that stomates can play a clear role in facilitating uptake of nutrients. For example, in a series of experiments with bean leaves where stomates were manipulated to be open or closed, uptake of nitrogen from urea, ammonium or nitrate was two times higher when the stomates were open. It should be noted that uptake via stomates does not just flow through the open stomatal pore; it is still a diffusion process along the edges of the stomatal guard cells that is more apparent when the pore is open. Additionally, recent studies using some new-age microscopic imaging methods showed that the base of trichomes was a key area for the uptake of foliar zinc in sunflower leaves, and other specific cell types directly above major veins was the primary uptake site of foliar phosphorus in barley leaves.
It is worth noting that much of what we know regarding cuticular transport of plant nutrients was derived from studies using isolated plant cuticles (enzymatically isolated from leaves.) Such a model system has its drawbacks, and artifacts can occur. For example, some studies comparing isolated cuticles to intact leaves did not yield consistent results for transport. This is why real-world studies with natural plant canopies under production conditions are needed to provide practical data on efficacy of foliar fertilizers. Numerous other factors not discussed here can also influence the success of foliar fertilization, which makes generalized recommendations difficult.
Foliar Nutrient Trials in Oregon Vineyards
My lab has conducted a number of foliar nutrient trials, and I will briefly share some of our findings. We have conducted three different trials using foliar urea-N for the specific purpose of boosting fruit or must YAN levels. Two experiments were conducted in Pinot noir and one in Chardonnay. These trials have shown that foliar urea is an effective tool to boost YAN (Table 2), and it has the advantage over soil-applied N in that canopy growth is not increased (a clear benefit for improving YAN in high-vigor sites.)
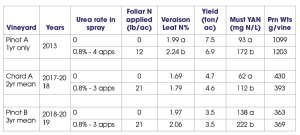
We also conducted a P trial years ago at two Pinot noir vineyards in the Willamette Valley. P was applied in three foliar sprays at a moderate dose, and leaf blade and petiole P status was improved at only one of the two sites. The increase in leaf blade P concentration at one site at veraison was only about 12%, and I concluded there was little benefit to spraying vines with foliar P (data not shown).
Currently, as part of the High-Resolution Vineyard Nutrition Management (highresvineyardnutrition.com/project), we are testing a foliar potassium treatment along with two rates of soil-applied K to correct a low K Pinot noir vineyard, and we are assessing two rates of foliar magnesium to correct a low Mg Pinot noir vineyard.
In the K trial, we are comparing a foliar K product (K-Metalosate, Albion Labs, Layton, Utah) at the highest label rate using three sprays per year (total = 3.3 pounds K/acre) with fairly high doses of soil K (K2SO4) at either 188 or 369 pounds K/acre applied in spring. Results from year 1 of this trial showed no increase in leaf or petiole K status at bloom or veraison, nor any impacts of K on growth or yield as well as must sugars, pH or TA. However, K levels in dormant-season canes (pruning wood samples) were slightly improved by all three K treatments compared to the no-K control. In year 2, leaf and petiole K status at bloom was increased in the soil K treatments, more so by the high rate, but not in the foliar K vines. Veraison leaf and petiole nutrients and pruning wood nutrients have yet to be analyzed for year 2, but similar to last year, no effects on growth or yield as well as must sugars, pH or TA were apparent due to K treatments (data not shown).
In the Mg trial, we are testing foliar sprays using MgSO4 (Epsom salt) because an earlier trial my lab conducted in the Willamette Valley with soil-applied MgSO4 at a high rate was unsuccessful. We are comparing foliar sprays of MgSO4 at 10 or 20 pounds/acre using three sprays (supplying a total of 3 or 6 pounds Mg/acre). The high rate of Mg increased leaf blade Mg levels at veraison in year 1 but did not alter petiole Mg levels as we found before. Both the low and high rates of foliar Mg reduced the number of leaves with Mg deficiency symptoms by more than 75% around harvest time in both years (Figure 1). However, Mg applications did not affect growth or yield as well as must sugars, pH or TA thus far.
In conclusion, foliar feeding of plant nutrients has its place in vineyard management, and certain foliar nutrients are effective in improving nutritional health of grapevines. This is especially true for micronutrients, notably B and zinc. Of the macronutrients, both Mg and urea-N have been effective in alleviating Mg deficiency or in boosting must YAN levels. Foliar applications of P and K have proven less effective so far. Caution is warranted when considering the myriad of foliar products that are being marketed to you as grape growers, and this is because we still have much to learn about how and when foliar-applied nutrients will be effective. A key question you should ask yourself when considering foliar nutrition is whether you have a deficiency or other problem that needs to be fixed. This is where money spent on tissue sampling for nutrients and tracking vine nutrient status over years will pay for itself in the long run. Prophylactic use of foliar fertilizers, except perhaps for B, is generally not a good idea.