It was in 1991, when I received a call by the President of the former California Pistachio Commission, Karen Reinecke, asking if there was a way to get involved as a technical member of the newly then established Aflatoxin Elimination Workgroup. The goal of this Workgroup was to evaluate proposals submitted by the United States Department of Agriculture (USDA) and University researchers to the USDA Special Fund to participate toward research to eliminate the problem of aflatoxin contamination by the year 2000. We are now in the second half of 2019 and aflatoxin was not only eliminated by 2000, but indeed contamination created major problems in some years in both pistachio and almond.
Our first proposal submitted in 1992 to the USDA/ Aflatoxin Elimination Technical Committee. It was funded and research started after hiring postdoc associate Dr. Mark Doster, a University of California (UC) Davis graduate with postdoc research at the University of Cornell. Mark an expert in fungal pathology and practical plant pathology was immersed quickly in this research. We were also supported then by the California Pistachio Industry which supplemented funding to intensify the research to reduce aflatoxins and find solutions for the growers. At the same time other researchers from UC Davis focused on aflatoxin research to reduce it in almond and walnut. Later on, we expanded our aflatoxin management research and included almonds and figs.
Aflatoxins are toxic compounds produced mainly by certain molds called Aspergillus flavus and A. parasiticus when these molds grow on various susceptible crops. These molds produce toxins that are considered toxigenic. The toxins produced are called aflatoxins which are considered as the most potent naturally-produced carcinogenic compounds causing liver cancer and in acute situations deaths. Strains of Aspergillus flavus that do not produce toxin are called atoxigenic, and they act as biological control agents. When they are applied on the orchard floor displace the toxin-producing mold strains, and reduce the potential for aflatoxin contamination in various crops.
There are four major aflatoxin types: the B1 and B2 produced by both the above mentioned fungi and G1 and G2 produced only by A. parasiticus. B1 is the most toxic among the four aflatoxin types. Because of this high toxicity these compounds are regulated strictly by various governments, and in fact, the B1 is regulated separately. For instance, in the USA the tolerance for B1 is 10 ppb (parts per billion) and for all the aflatoxins (total) is 15 ppb. The European Union (EU) has even stricter tolerances, i.e., 8 ppb for B1 and 10 ppb for total aflatoxins. One can judge the seriousness of aflatoxin contamination not only from the very strict tolerances but also from the losses and additional costs associated with the re-sorting and losses associated with the dumping the contaminated product. It should be noted that when shipments exceed the threshold, the consignments are rejected and must either be reconditioned or destroyed.
Below is a historical summary how this technology developed to help our California nut crop industries and the fig industry.
In the first eight years we focused on cultural practices that affect the predisposition of the pistachio crop to aflatoxin contamination and also find out whether contaminated nuts show special characteristics that can be used to sort out these nuts at the processing plant.
Below is a list of the findings from those studies led by Drs. Doster and Michailides:
- We confirmed that early split nuts (ES) contained large amounts of aflatoxins and we named these nuts the “Achilles Heel” for aflatoxin contamination.
- ES by themselves explained 84 percent of the aflatoxin contamination in the samples we analyzed.
- When the ES were combined with the navel orangeworm (NOW) damaged nuts explained 99 percent of the aflatoxin contamination in the samples we analyzed.
- We reduced the incidence of ES by providing the trees with sufficient water during early season (May) when it is the critical time for the full size development of nut shell (water stress of trees during May leads to higher incidence of ES).
- We compared the incidence of ES on Kerman under the influence of four rootstocks: UCB1 and Pioneer Gold I resulted in significantly lower ES incidence than Pistacia atlantica and Pioneer Gold II rootstocks.
In my first Aflatoxin Elimination Committee (AEC) Meeting in 1991, in Peoria, Illinois, I learned for the first time that some strains of Aspergillus flavus do not produce aflatoxins and some USDA researchers reported that a very large portion of the A. flavus population consisted of strains that do not produce any aflatoxin, called atoxigenic strains. They started using these atoxigenics as candidates for biological control of aflatoxigenic fungi. It was also discovered that the proportion of strains that produced aflatoxin included strains that produced variable amounts of aflatoxins. USDA researchers started first working with atoxigenic strains to be used as biocontrol agents to reduce aflatoxins in the various crops. One of this strains was initially selected in Arizona from samples taken from cotton fields, and the Cotton Research Council of Arizona that supported financially this research were able to get AF36 registered for use in cotton and corn in 2008. Meanwhile starting in 2002, we discovered the same strain was the most commonly encountered strain among the other atoxigenic strains in pistachio, almond, and fig orchards in California, and immediately included this strain in our aflatoxin biocontrol studies. With multiyear support of USDA funding and funding by the pistachio and fig industries in California, we were able to show that this strain is among the most common atoxigenic strains occurring in California nut crop and fig orchards, and indeed it can be found at much higher incidence in comparison with all other atoxigenic strains. For instance, during these studies 15 different groups of atoxigenic strains were determined, and each one was at a rate of less than one percent, while the AF36 atoxigenic strain (Figure 1) was found in an average of five to eight percent depending on the field and the type of the crop, and in some instances up to 12 percent of populations of the atoxigenic strains
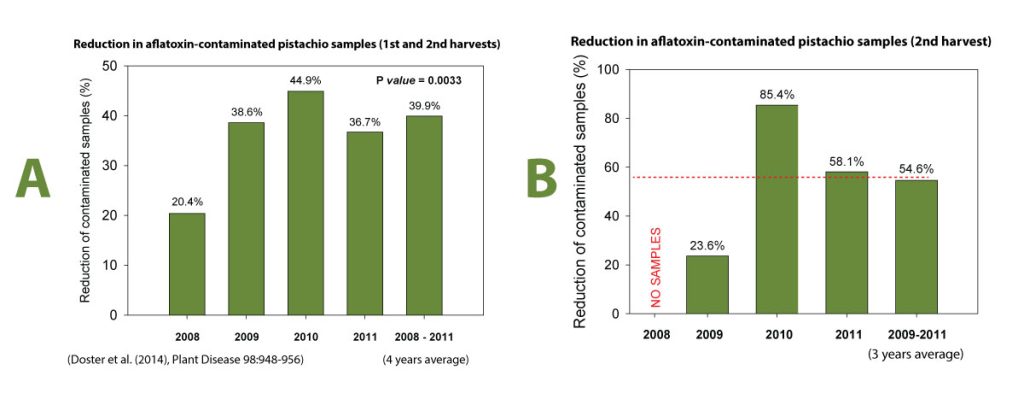
Initially, the studies were confined in small experiments in replicated micro-plots where we showed that when the AF36 was applied once preseason, it persisted well until the next application in the following year and displaced the toxigenic A. flavus strains at a rate of 90 to 95 percent displacement. These results, the fact that this strain was native to California orchards, and it was the most common atoxigenic strain and the toxicological data developed by the USDA in Arizona were sufficient to submit to the Environmental Protection Agency (EPA) to request an experimental use permit (EUP) to test the strain commercially on a larger acreage without the requirement to follow crop destruct requirements as needed with application of experimental compounds. The EUP was approved in 2008 and 3,000 acres of pistachios were treated with the commercial product of Aspergillus flavus AF36 strain produced in the Cotton Council of Arizona facility in Phoenix, Arizona. Also, 3,000 acres of pistachio close to the AF36-treated orchards were used as untreated controls. All this acreage for the EUP was provided by the former Paramount Farming Company (now Wonderful Orchards Company). At harvest the treated and the untreated fields were sampled separately and the samples, specifically called “library samples”, were analyzed for aflatoxins. For four years we showed a significant reduction of aflatoxins (Figure 2A). The average of this reduction for the four years of the EUP was close to 40 percent. When library samples of reshakes were analyzed for aflatoxins, this reduction in one year reached to 85 percent (Figure 2B). These commercial efficacy data were sufficient to obtain registration of AF36 for use in pistachio in the states of California, Arizona, Texas, and New Mexico. After the registration of AF36 on pistachio, the Almond Board of California and the California Fig Institute (the latter has funded research during the early stages of our aflatoxin research) became interested in completing any additional research so that the AF36’s registration is expanded to include almonds and figs.
It took 5 years of additional research (funded by the Production Research and Food Safety and Quality Committees of the Almond Board of California) to provide the USEPA and the California of Pesticide Regulations Department the additional data for the registration of AF36 for use on almond and figs. Meanwhile because the manufacturer of the commercial product changed the initial wheat carrier of the AF36 strain to sorghum (Figure 3) the product was registered as AF36 Prevail® in January 2017 and included all, pistachio, almond, and fig. Although the pistachio industry adapted the use of AF36 widely and from 75,000 acres treated in 2012 reached to up to 200,000 acres in 2018, the almond industry was a little hesitant in widely adopting this new technology, despite the fact that there was good efficacy in reducing aflatoxin contamination in pistachio orchards. We expect to see better efficacy when all pistachio, almond and fig orchards are treated on an areawide basis because the spores of the biocontrol can spread from field to field easily with even slight windy conditions and dust.

Description of the Biocontrol Product
Initially the AF36 product used sterilized wheat as the carrier. Sterilized wheat seed was inoculated and incubated under certain favorable conditions for the strain to invade and grow in the entire wheat seed. More recently and after additional studies in Arizona it was determined that sorghum, which has a lower cost was as good carrier as the wheat (even better under conditions in cotton and corn fields) and the manufacturer replaced the wheat with sorghum and at the same time changed the method of inoculations. The sorghum seed now is coated with a mix of a polymer and spores of AF36 instead of waiting for the seed to be colonized by the atoxigenic mold. This was done in order to satisfy the increased demand for tons and tons of inoculum since the rate is 10 lbs per acre. Studies in California under orchard conditions indicated that the sporulation on the sorghum seed is delayed, although by the end of a week both sorghum and wheat showed similar sporulation rates. The time of production of spores and the rate of sporulation are very critical for the successful use of this biocontrol agent. It is a numbers game: we want to overload the soil with atoxigenic spores and displace the spores of the toxigenic molds. That is the way this biological control approach works in the field (see challenges at the end of this article relevant to sporulation).
Proper Way for Ground Application:
For detailed information please read the label of the product:
Michailides emphasized that as far as we know up to now there are four critical application factors that need to be taken into account for AF36 Prevail to be successful in the orchard: a) the timing of application; b) the rate (amount) per acre; c) the proper placement on the orchard floor; and d) the proper irrigation before and after the application.
- Timing for pistachio, almond, and fig: Apply Aspergillus flavus AF36 to the surface of the soil under the plant canopy with a granular applicator and do not cover the AF36—colonized grain with soil. For pistachios apply the product from late May through July, for almonds from late May to early July, and for figs from early May to early June. Specifically in almonds, application should be timed around hull split. If you know when to expect hull split, you should time application about one to two weeks before. You want to have the max sporulation of the biocontrol during the hull split stage of the nuts.
- The rate (amount): The proper application rate is 10 pounds per acre. A single application should be made each year.
- Proper placement: AF36 Prevail should be applied within the berm area of the orchard, not at row middles, so that it will be reached by the irrigation system and minimize delivery to areas that do not get wet.
- Proper irrigation: Irrigation is required directly after application. Irrigation within three days after application of Aspergillus flavus AF36 will improve efficacy. The AF36 product will not sporulate without moisture and can fail if there is too much moistureAim for soil moisture levels around 13-18 percent. Proper placement within the berm, close to the irrigation system, will ensure it is successfully activated.
- “Conditioning” of the orchard floor before application: This is practice that some growers have figured out on their own. Pre-irrigating and then about two days later apply the AF36 inoculum and then apply irrigation as in (d) above. Although Michailides and his crew do not have any data to support this practice, they strongly believe the practice of pre-irrigation will speed up the sporulation of the product since the rehydration can start as soon as the product comes in contact with the pre-wetted soil.
A 45 Percent Reduction of Aflatoxins is a Reality Now
Dr. Themis Michailides, plant pathologist at the UC Davis/Kearney Agricultural Research and Extension Center and former member of the National Aflatoxin Elimination Technical Committee, and Dr. Mark Doster have devoted decades to studying aflatoxins and their work has been instrumental in development and registration of AF36 in pistachio, almond, and fig.
Until now, Michailides notes, tree nut and fig growers had no direct way to combat aflatoxin. Instead contamination has been managed primarily through preventing navel orangeworm damage. While as noted below, effective orangeworm management is still very critical and essential in reducing crop damage, AF36 offers an additional tool that has a direct impact on reducing harmful toxigenic Aspergillus mold strains and the aflatoxin they produce.
Recent Challenges with the use of Aspergillus flavus AF36
AF36 Prevail can result in more than 80 percent reduction of aflatoxin contaminated cotton and corn, but here in California, only once we reached an 85 percent reduction (Figure 3) and this was only in the second harvest (reshakes) pistachios, which have higher risk for NOW infestation and aflatoxin contamination than the pistachios of the main (first) harvest. Results of analyses of a large number of library samples obtained from treated and untreated fields of Wonderful Orchards Company, resulted in a significant reduction of up to 45 percent in aflatoxin contamination. Michailides’ lab crew is trying to explain why the efficacy of AF36 in reducing aflatoxin cannot match the one reached in cotton fields in Arizona and corn fields in Texas. The following challenges may explain partially these disadvantages of the AF36 product in California: a) inadequate soil moisture and temperatures; b) not correct timing of application; c) delayed harvest, and d) inefficient control of NOW, both contributing to increased aflatoxin contamination; e) arthropod pests of career seed; and f) other predators (rodents, birds, etc.). Recent research projects are focused on addressing all these challenges. One new development is that a new product (Afla-Guard®, manufactured by Syngenta Company) that is registered to reduce aflatoxin contamination in peanuts and corn was introduced in California for experimentation and gathering data to support registration for use in pistachio, almond and fig. If successful, this will be the second product registered in the USA which represents a different from the AF36 atoxigenic strain of Aspergillus flavus and the carrier is barley (Figure 4). Studies done by Drs. Jaime and Michailides (Kearney Agriculture Research and Extension Center) in 2017 and 2018 showed that this product sporulated faster, better, and under lower temperatures and lower soil moisture content than the Aspergillus flavus AF36 strain. Registration of this product in California is anticipated in 2020, so that growers may then have a second tool to choose to combat aflatoxin contamination in 2020.

NOW Management is Still Essential
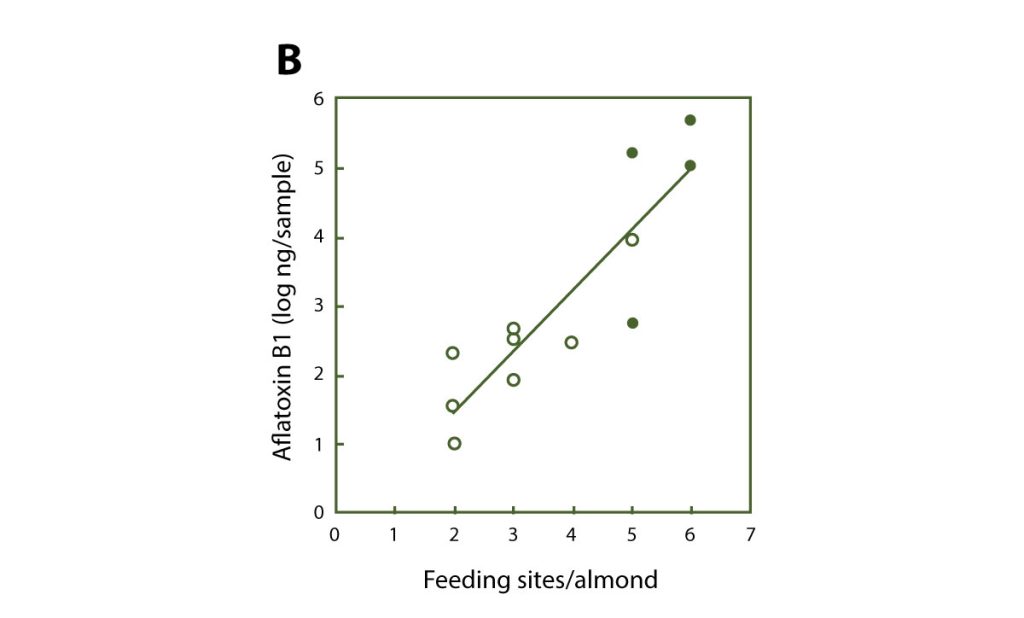
Aflatoxin contamination becomes a major problem in years when damage by navel orangeworm is higher than the standard low level. Relatively studies by Drs. Michailides and Palumbo (ARS (Agricultural Research Service), USDA, Albany, CA) showed that NOW moths are heavily contaminated with spores of aflatoxigenic fungi as soon as they emerge from mummies in early spring. Also as the damage on nuts increases so is the incidence and the amounts of aflatoxins (Figure 6). Therefore, it is essential to keep up with navel orangeworm pest management practices. Growers should use all the available tools for reducing damage by NOW to supplement the mummy sanitation, which should be the first step towards aflatoxin reduction. Reduction of NOW damage can also be achieved by timely harvest, in season insecticide sprays, and winter mammy shake.
Where to Find the Product, Learn More, and Application Services
To learn more about AF36 and its application, watch two short videos produced by California Pistachio Research Board. Although they were filmed in a pistachio orchard, the information is useful and accurate for almond and fig growers.
Western Milling is the distributor for AF36 in California. Growers can contact Jeff Chedester, seed business manager, at (559) 302-2593; and Agri Systems, Inc., c/o Brendan Brooks, at 559-665-2100 for product information and application. Distributors for the second product will be provided as soon as it is registered in California.
Acknowledgments:
The author thanks all his collaborators for the dedicated research, the California Pistachio Industry (California Pistachio Research Board), the Almond Board of California, and the California Fig Institute for their continuous financial support of these studies. We also thank the USDA the initial seed grants provided by the Aflatoxin Elimination Technical Committee, and California Department of Food and Agriculture (CDFA) (Grant SCB16054). Special thanks go to Wonderful Orchards for their continuous support of this research by allowing using their orchards for various experiments; also we thank Keenan, Sutton, and Nichols Farms for their support as well. In addition, we appreciate very much the support by Syngenta in 2018 and 2019.


















