Phosphite has been a controversial topic for years. Its use and benefits are argued in hundreds of research papers across the world’s scientific communities. Is it a fertilizer, a biostimulant or a fungicide? These questions are discussed in multiple university research results. I believe that if we look carefully, we can conclude phosphite serves all three functions. As with everything we do with chemicals and nutrition, we need to be aware of possible negative effects. We also need to determine how we are using the phosphite materials and the results we are seeking.
First, we need to understand and differentiate between phosphate and phosphite. There is often a lot of confusion here.
It Starts with Phosphorus
Phosphorus (P) is an essential major macronutrient. It is required by all living organisms. It is a limiting nutrient that controls growth in many ecosystems. P in living systems occurs mainly in the form of inorganic phosphate as well as phosphate esters. The formation and breakdown of phosphate esters under the control of kinases and phosphatases regulates the temporal protein activity and is responsible for the generation, distribution and utilization of free energy throughout the cell in several metabolic pathways. P is a structural component of the phospholipid bilayer membranes and genetic material including DNA and RNA. In nature, most P exists in its completely oxidized state (valence of +5) as a phosphate anion (PO43-, Pi), phosphate-containing minerals and organic phosphate esters. Pi compounds are the only form of P utilized by the plants for their nutrition. Modern agriculture is currently dependent on the continuous input of Pi fertilizers, produced by the mining of rock Pi. Approximately 80% of the mined P is used to manufacture Pi fertilizer.
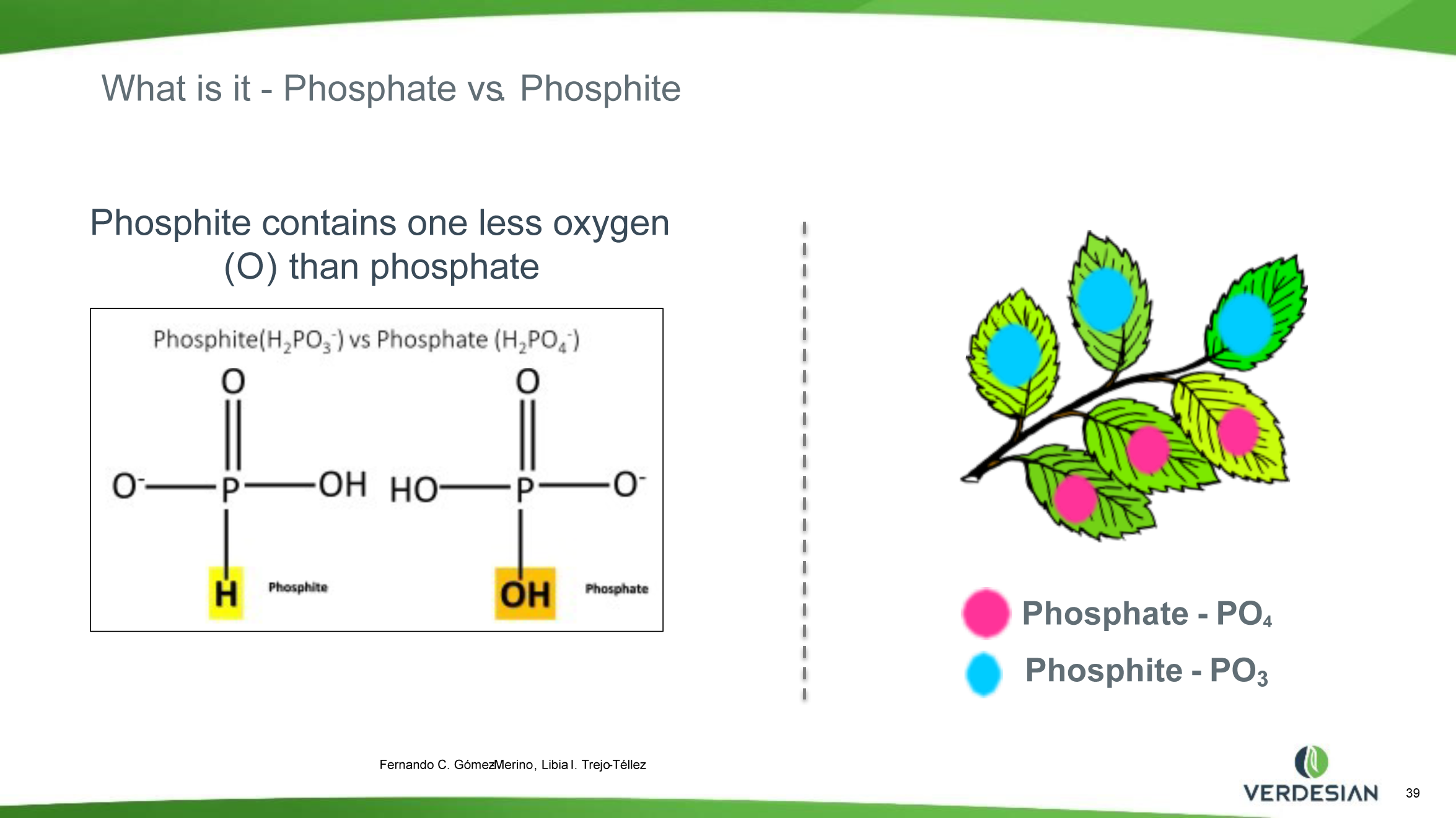
The Difference is An Atom
Phosphite (Phi) is a reduced form of Pi with one less oxygen atom (P valence of +3). Phi compounds have been widely used in agriculture as fungicides for controlling several plant diseases caused by oomycete pathogens including Phytophthora spp. Although Phi can be absorbed by the plant cells through the Pi transporters, plants cannot metabolize Phi, which limits its use as a fertilizer. Phi can only be metabolized naturally by certain bacteria with an enzyme called Phi dehydrogenase (PtxD), which oxidizes phosphite into phosphate, a form that can be utilized for various cellular functions, and it does not provide P nutrition to the plants. The supply of Phi attenuates the Pi starvation responses (PSRs) in plants and inhibits the growth in Pi-starved plants. The addition of Phi to plants experiencing deficiencies of P confuses the plant’s internal signal that triggers the P deficiency responses. Several studies show that too much Phi application has detrimental effects on the growth and development of various plants. Therefore, Phi compounds should not be used as a major source of P fertilizers in agriculture.
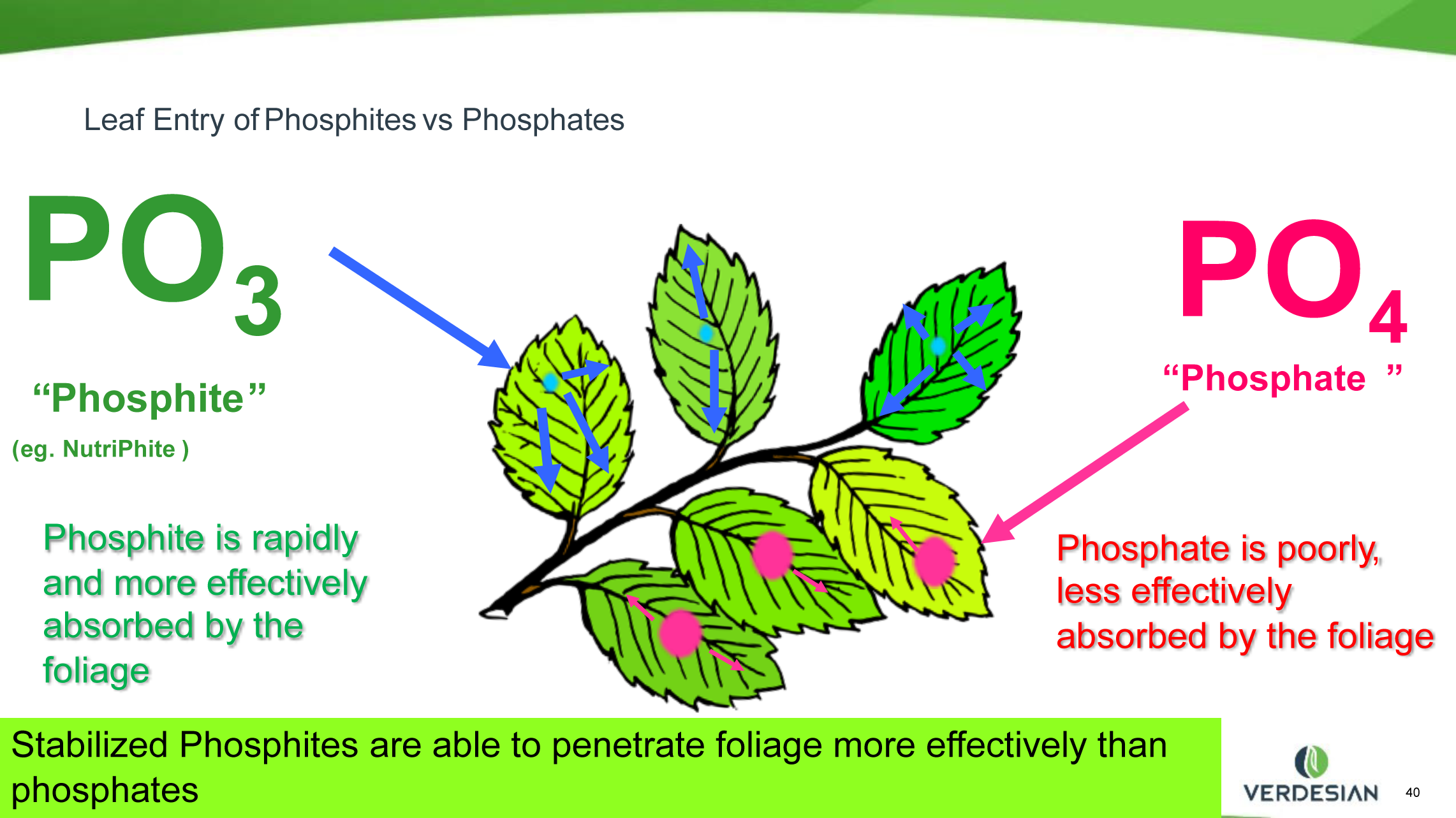
Phi is more soluble than Pi and has a smaller structured molecule, so it is more readily absorbed by plant tissue. When added with other nutrients, it can serve as an excellent carrier. For example, a Phi mix containing phosphorus and potassium, calcium or magnesium would be absorbed readily and carry the needed nutrient into the plant. This has been demonstrated in numerous trials based on Verdesian Life Sciences’ line of NutriPhite nutrient products. Because of the proven biostimulant properties of Phi and the fungicidal benefits, we have the possibility of getting multiple benefits applied with a single application.
Phosphite Not a ‘Traditional’ Fertilizer
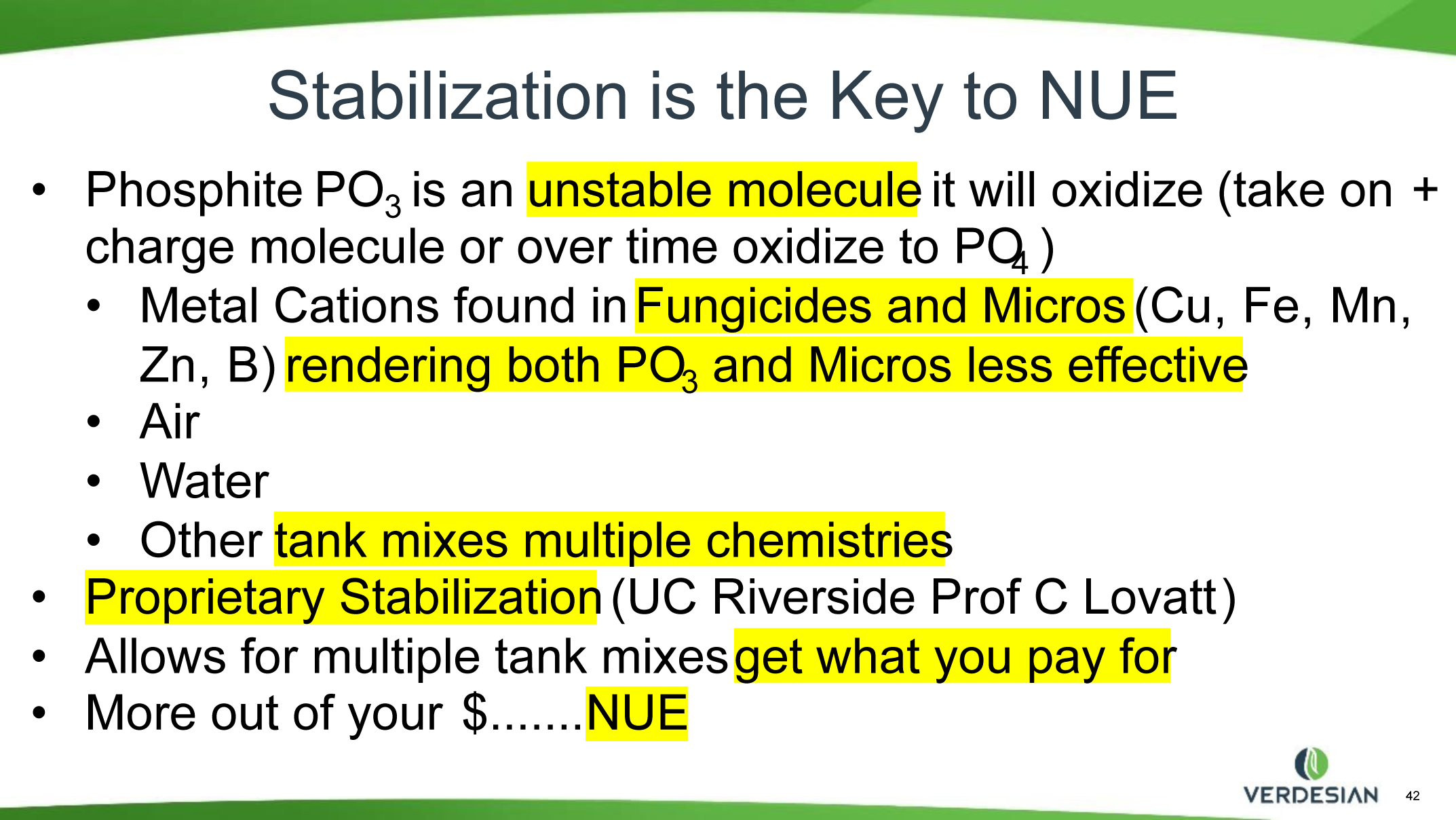
So, though Phi is not the best nutritional source of P, it can serve as a carrier and catalyst for quick nutrition supplements and responses to nutrient deficiencies. Soil applications of Phi usually do not replace P in the soil; however, plantings the following year show the plants do better where Phi had been applied the previous season. Interest in using Phi as part of a total production package is increasing, especially for some high-value crops. Phi fertilizers, if not formulated correctly, have significant potential to be phytotoxic and induce adverse reactions with other materials in the spray tank such as microelements and pesticides. When choosing your Phi source, it would be wise to seek a stabilized formula as it is proven not to bind with other tank mix materials. Chemical bonds will create another compound that even if not phytotoxic can be completely useless and unattainable by the plant. The salt-out effect can be a potential to clog nozzles and filters and could result in a waste of dollars spent on the Phi and/or other nutrients and chemicals. By binding these up, we are losing all or most of the efficacy of an expensive input in our crop management. All fertilizers, especially Phi, should be used in close consultation with a crop consultant to meet desired production goals.
In California, it is important to recognize that research has shown foliar applications of Phi can replace Pi in citrus and avocado crops suffering from P deficiency. Phi conversion to Pi may be attributed to slow chemical oxidation or by oxidizing bacteria and fungi that have been found living on the leaves of these two crops.
In part two of this article series, we will visit the biostimulant role of Phi, which is possibly even more important than the fungicidal properties of this very diverse and very useful tool to consultants and growers.