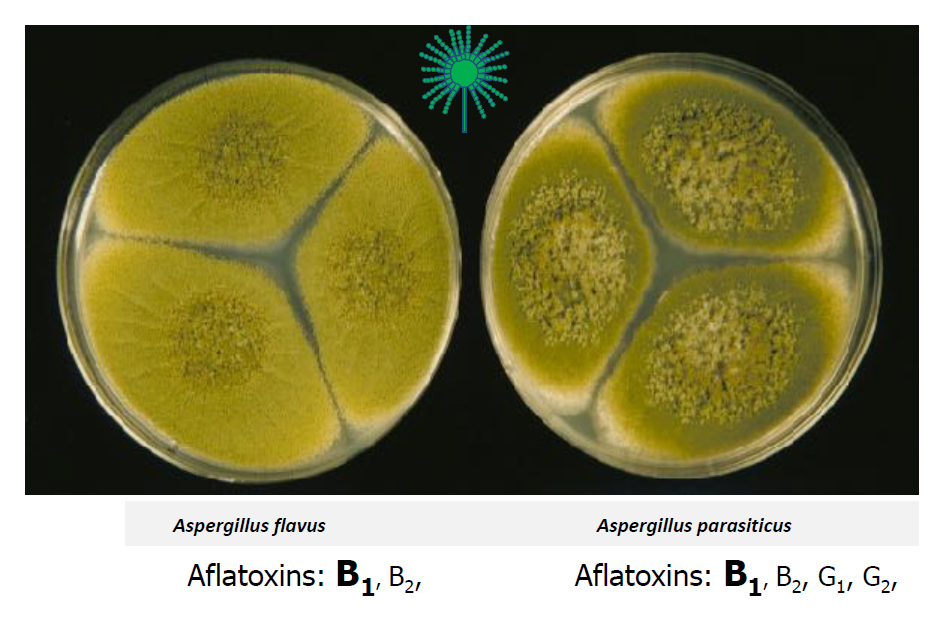
Aflatoxins are a category of mycotoxins, which are toxic compounds produced by fungi (Myco= µύκης, which means fungus in Greek.) So, aflatoxins are toxins produced by specific types of fungi. These compounds are by-products (secondary metabolites) produced mainly by two fungal species, Aspergillus flavus and Aspergillus parasiticus, which are present in California nut crops and fig orchards when they grow in susceptible substrates. These compounds are very toxic, carcinogenic and cause disease when consumed in large amounts. Because of the high toxicity, aflatoxins are regulated worldwide by governments, dealing with marketing products susceptible to contamination with these mycotoxins. There are several kinds of aflatoxins, such as B1 and B2, produced by the above Aspergillus species. In addition, the latter species produces two more kinds of aflatoxins, G1 and G2. The letters “B” stands for the blue color that these compounds show and the “G” for the green color when contaminated products are placed under a UV light (365 nm wavelength).
In the U.S., the tolerance for aflatoxins is 20 ppb (1 part per billion =0.000000001 gr). In the European Union (EU), for all (total) aflatoxin, the tolerance is 10 ppb while for B1 is 8 ppb, and these values are for pistachios and almonds for direct consumption. The tolerances are even stricter for walnuts and dried fruit (4 and 2 ppb for toral and B1 aflatoxins, respectively), and for dried figs, total aflatoxins are 10 ppb while for B1 is 6 ppb.
Because of these strict tolerance thresholds, the U.S. (and to a lesser degree other countries that produce and market products susceptible to aflatoxins) takes extraordinary measures to reduce this contamination as much as possible.
Early Aflatoxin Research
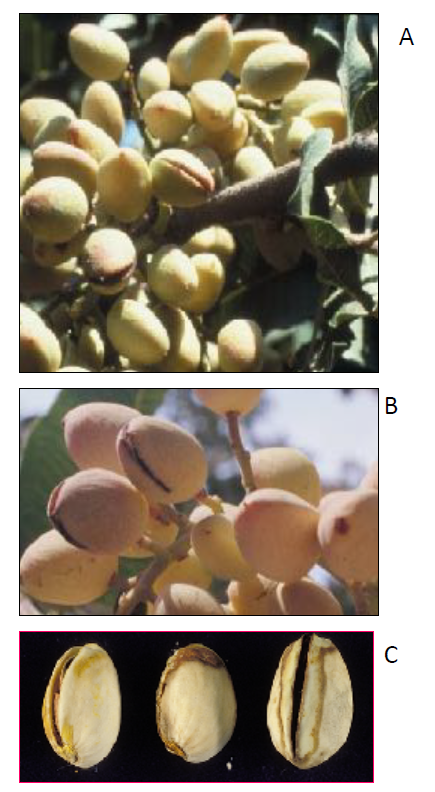
For a decade (1991 to 2001), the research in our plant pathology laboratory focused on cultural practices and reduction of damage by the navel orangeworm (NOW) to reduce aflatoxin contamination in almond and pistachio. For instance, in early research on pistachio, we discovered that the early split (ES) nuts (Figure 1) serve as the “Achilles Heel” for infestation by NOW and infection by aflatoxigenic fungi, leading to aflatoxin contamination.
In fact, when samples were collected from several orchards and separated into ES without NOW damage, ES with NOW-damaged nuts and normal nuts (mature nuts but having hulls intact) and analyzed for aflatoxins the results showed that ES contribute a lot in aflatoxin contamination of pistachio as follows: ES (shriveled, i.e., those that had developed early and infested by NOW) had an average of 84% of the total aflatoxins in these samples; if one includes the ES with no NOW infestation, the total levels of the aflatoxins could explain 99.9% of aflatoxins in these samples. Although 20% of the samples represented ES that developed close to harvest had aflatoxins, the levels were only 2 ppb, representing only 0.1% of the total aflatoxins.
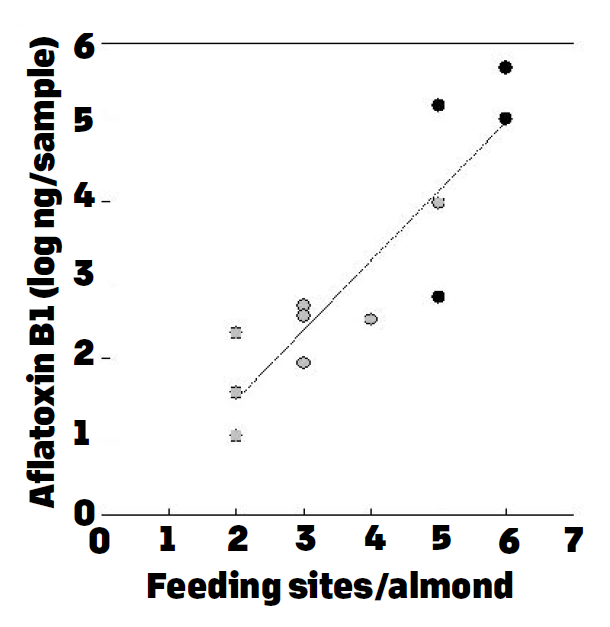
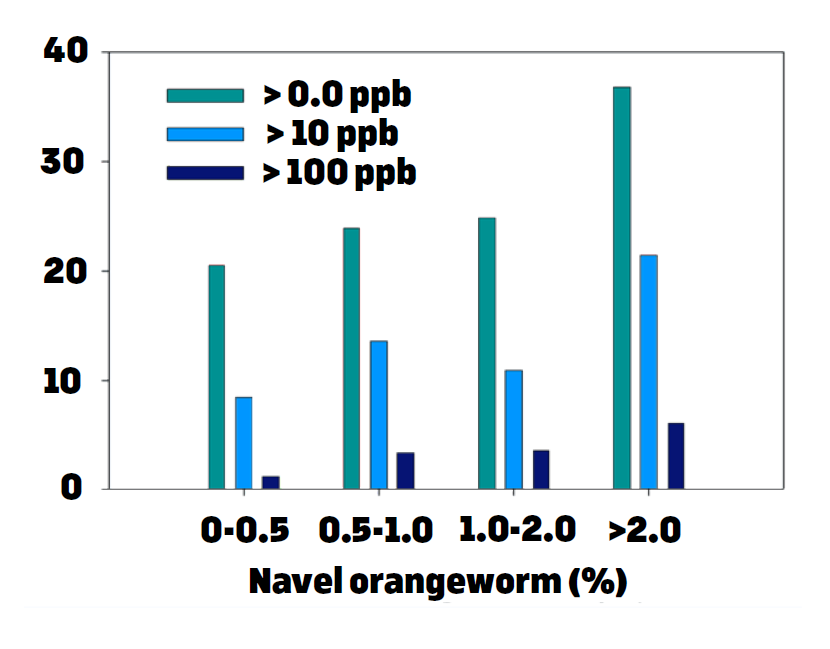
These results made us investigate ways to reduce the incidence of ES and also methods to reduce NOW damage. Normal mature nuts had no aflatoxins in these samples. The results were very consistent in two consecutive years. Moreover, when samples were collected and were separated based on their levels of NOW damage, we showed that as the level of damage increased, so did the aflatoxin contamination frequency and amounts (Figure 2). Similarly in almonds, as the feeding sites caused by the NOW larvae increased on almond kernels, so did the aflatoxin levels (Figure 3). Therefore, it is obvious reducing the damage by NOW will result in lower risk for aflatoxin contamination, and this is accomplished by doing sanitation (“mummy shake”) in both almond and pistachio. Avoiding water stress is another approach to reduce susceptibility of trees to aflatoxin contamination. Specifically in pistachio, water stress in early season (May) increases the incidence of ES, and thus increases the risk for aflatoxin contamination.
Biological Control
Because aflatoxin contamination is very sporadic, and because there are no fungicides that would affect aflatoxin contamination, several countries now emphasize a biological control approach by displacing the toxigenic strains of A. flavus and A. parasiticus with the use of atoxigenic strains that are applied directly on to the orchard floor. In California, in cooperation with Dr. Peter Cotty (USDA-ARS), the A. flavus AF36 strain was registered first in 2012 for use in pistachio and then the same product as AF36 Prevail® in 2017 for use in pistachio, almond and figs. As of August 2021, a second product using a different atoxigenic strain, Afla-Guard® GR manufactured by Syngenta Crop Protection, LLC, was registered for use in almond and pistachio. Pistachio and almond growers can use either product starting in the 2022 growing season.
But before moving into the application of either of these products, we need to provide a brief background into the history how this technology had developed over many years of research and the various challenges orchardists have experienced over the years in using the AF36 and/or the AF36 Prevail products.
As mentioned earlier, there are two major fungi that can produce aflatoxin and contaminate the susceptible crops, A. flavus and A. parasiticus. The A. flavus produces two strains in the soil based on the size of the sclerotia (resistant, survival structures), the S strain and the L strain (Figure 4). All the isolates of the S strain are toxigenic, while among the isolates of the L strain, there are strains that produce various levels of aflatoxins and others not producing any strains. These latter strains are called atoxigenic.
All the strains of A. parasiticus are aflatoxin producers. An atoxigenic strain called AF36 was found to be among the most frequently encountered atoxigenic strains ranging in incidence from 4% to 12% while other groups of atoxigenic strains ranged from <1% to 2% among the strains in the soil. This strain was determined to be the same that was isolated earlier in Arizona from cotton fields and used there as a biological control agent to reduce aflatoxin contamination in cotton and corn. Therefore, since all the toxicological studies were done by USDA and because the AF36 was the one most frequently encountered in California tree nut and fig orchards, we selected and used it as a biological control agent in the Kearney experimental orchards first to measure displacement of the toxigenic strains.
After 10 years of research in microplots initially and three years as an Experimental Use Permit compound used in 3,000 acres of pistachio, eventually, the AF36 strain was registered for use in pistachio orchards in 2012. The initial carrier of the strain was wheat seed inoculated in big fermenters with the A. flavus AF36 strain. Five years later, the manufacturer was able to register the AF36 Prevail® product, which contains the same AF36 strain and uses sorghum as the carrier, the surface of which is coated with propagules of the atoxigenic strain via a polymer. Recently, a second atoxigenic strain, Afla-Guard GR, was provisionally registered for use in pistachio and almond. There is also an interest now by the walnut and fig industries to have both these products registered for these crops.
Challenges
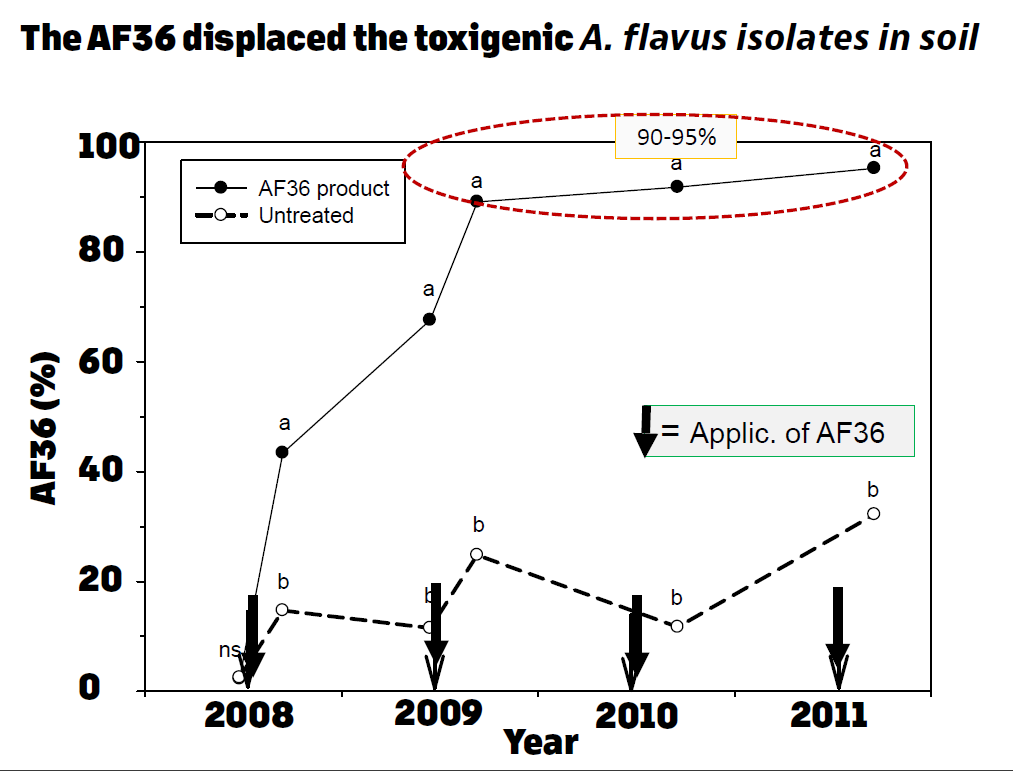
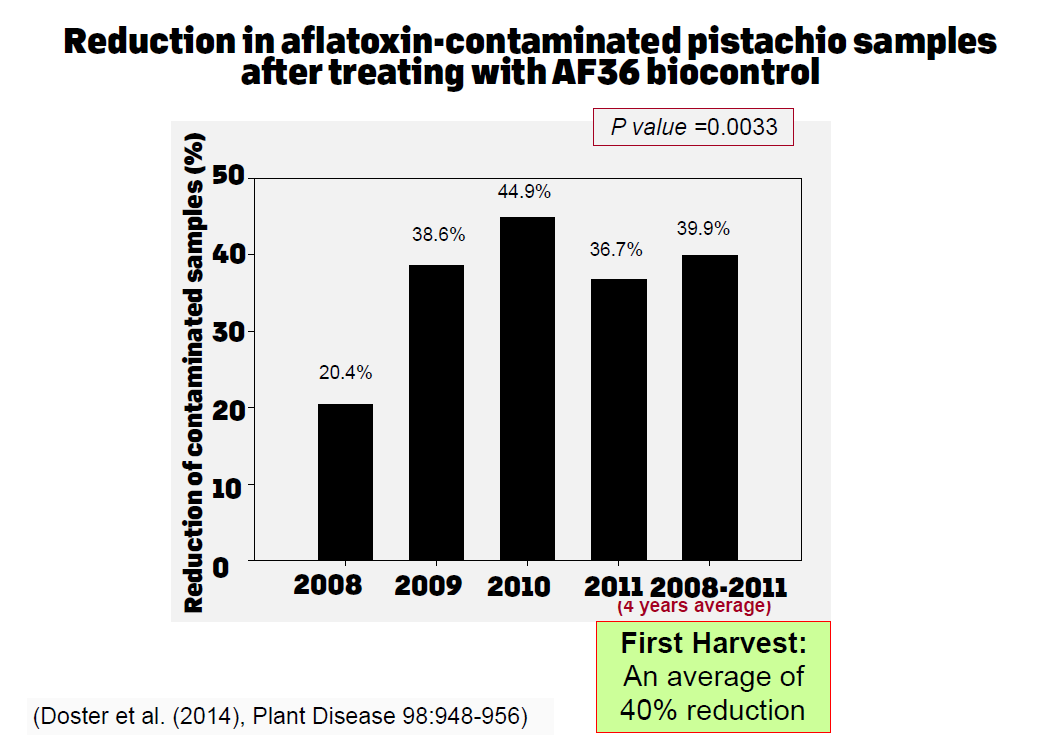
When experiments using the atoxigenic strain AF36 were initiated in 2002, we determined a very high displacement ability of the toxigenic strains in the soil of microplots, which reached almost to 95% displacement at the end of the fourth year, applied once per year. When the EUP was granted in 2008 and 3,000 acres of pistachio were treated with AF36 once per year, the displacement also reached to 95% by the fourth year of application (Figure 5). It was during this time when analyses of first harvest pistachio library samples showed a 40% reduction in aflatoxin-positive samples and an almost 55% reduction in aflatoxin-positive library samples of the second harvest (or reshakes) (Figure 6).
During 2012 to 2019 and after application of the product yearly, we noticed a lower percentage of displacement that ranged from 50% to 70% (Figure 7). The blocks that were treated last in 2011 showed a continuous decrease of displacement from 65% down to about 30%, which remained stable from 2015 to 2017, but there was a jump to almost 50% in 2018 (Figure 7). The untreated fields ranged from about 32% to 20% during this period (2012 to 2018). During the same seven-year period, when library pistachio samples were analyzed for aflatoxins, only in two years (2013 and 2017) were there major reduction level in aflatoxin-positive samples, while in the remaining years, the reductions in positive samples were very small, ranging from 5% to 30%, and in some years (2014 and 2016), there was no reduction at all (Figure 8).
To overcome these challenges and to minimize the influence of treated orchards with AF36 Prevail® by toxigenic propagules (spores) escaping non-treated orchards, we initiated research projects funded by both the pistachio and almond industries on long-term areawide projects. The rationale was that when large area is treated year after year, the influence by non-treated orchards should be minimal. By doing this, we were able to increase the levels of displacement from about 45% in almond and about 65% in pistachio to 80% in almond and 95% in pistachio (Figure 9). This is a significant increase in the levels of displacement of toxigenic strains and we expect now to significantly reduce the levels of aflatoxin-positive samples. A good example for displacement of toxigenic strains is from our research with almonds, where toxigenic strains ranged from 65% to 83% before treatment with AF36 Prevail to about 25% toxigenic Aspergillus in fields treated, while the untreated orchard maintained an incidence of 68% of the toxigenic Aspergillus strains. In the treated orchards, the atoxigenic strains reached a level of about 80% as measured at harvest time. (Figure 10).
Moving Forward
The new biological product, Afla-Guard GR is now registered and will be available to pistachio and almond growers to use commercially in 2022. The active ingredient of Afla-Guard GR is the NRRL 21882 Aspergillus flavus strain and the carrier is barley seed. In contrast to AF36 Prevail that is sorghum seeds coated with the spores of AF36 strain of A. flavus, the NRRL 21882 strain is inoculated in the barley seed, i.e., the entire barley seed kernels are “infected” (colonized) by the latter strain.
The Afla-Guard GR product sporulated at higher rates under lower temperatures and lower soil moisture than the sporulation produced by AF36 Prevail when the two strains were compared side by side and under the same conditions. However, since we do not have any results from aflatoxin analyses of pistachio samples, it is still unknown how this product will perform under commercial application. The efficacy of each AF36 Prevail and Afla-Guard GR will be studied in side-by-side commercial fields this year for the first time.
In addition, we are doing studies to overcome other challenges with the commercial application of these products, such as managing predation by ants, rodents and birds, the negative effect of the direct sunlight on the seed inoculum under very high temperatures, the coordination of irrigation either just before or immediately after applying the biocontrol product, etc. All of these are challenges that can differ from orchard to orchard and therefore may affect variably the efficacy of the biocontrol products.
(Co-Authors of this article include: Mark Doster, Plant Pathologist, Pummi Singh, Post-Doctoral Research Associate, Giuseppe Fiore, Graduate Student, University of Bari, Italy, Victor M. Gabri, Staff Research Associate and Graduate Student, Yong Luo, Project Scientist, Ryan Puckett, Greenhouse/Cold Room/Physical Plant Officer and Laboratory Assistant, and Apostolos Papagelis, Agronomist, University of Athens, Greece, John Lake, Laboratory Assistant. All personnel are affiliated presently with UC Davis/Kearney Ag Research and Extension Center, Parlier, Calif. 93648.)
We thank the California Pistachio Research Board and the Almond Board of California (Food Quality and Safety Committee) for funding this research. Also, funding was provided by the USDA/Aflatoxin Elimination Technical Committee and the CDFA/Grant 16-SCBGP-CA-0035. Especially, we acknowledge the extraordinary support of Wonderful Orchards Company, which provided multiple and large-acreage sites for experimentation and hundreds and hundreds of library samples for aflatoxin analyses. It would not have been possible to complete this work and get registration of the atoxigenic strains of Aspergillus flavus without such immense support by this company and its dedicated personnel.