Johnsongrass (Sorghum halepense L.) is a perennial grass weed that has troubled California weed managers since the late 19th century, particularly in perennial systems where mechanical control options are limited. Johnsongrass infestations can be difficult to eradicate because the plant spreads through underground stems called rhizomes and abundant seed production from mature plants. Johnsongrass seedlings resemble corn seedlings and grow rapidly. Flowering stalks can reach six feet above ground and can produce hundreds of seeds each. Dormant seeds can survive for at least six years, and some seeds can survive ingestion by birds and mammals. Herbicides are the main tools used to manage johnsongrass in orchards and vineyards. Many preemergent herbicides can control johnsongrass seedlings but are ineffective on perennial plants. However, the discovery of glyphosate has led to powerful systemic herbicides that can provide good control of seedlings and those troublesome johnsongrass rhizomes. Despite this being the case, johnsongrass is still not easily eradicated once it has a strong foothold.
Are There Any Herbicide Resistance Concerns?
Glyphosate herbicides like Roundup are the primary tools used to eradicate pesky johnsongrass infestations, and efficacy has been reliable in California. However, elsewhere in the United States and worldwide, several cases of glyphosate resistance have been identified. In all cases of glyphosate-resistant johnsongrass that have been published, the reduction in control is due to a reduced effect of the herbicide on roots rather than the foliage. This happens because the herbicide applied to the leaf or stem is not transported into the roots and rhizomes in the quantities that would normally be expected. So in a resistant population, the aboveground portion of the plant can be fully controlled, but the rhizomes are relatively unaffected. This type of resistance is rather insidious and can be difficult to detect. Again, this phenomenon has not been identified in California johnsongrass populations, but weed managers ought to keep in mind that this species has a proclivity toward this form of resistance.
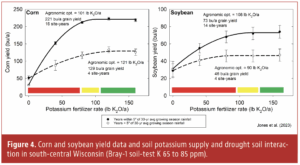
‘Only plots that received rimsulfuron in spring had any significant reduction in johnsongrass coverage or biomass.’
Post-Emergent Herbicides for Johnsongrass Control
Resistance management principles like herbicide rotation and tank mixing should be followed even when resistance has not been identified or suspected, so we should review the options available for chemical control of johnsongrass.
Three groups of herbicides are available for systemic post-emergent control of johnsongrass:
• Glyphosate-based herbicides (Group 9)
• Grass-selective herbicides like clethodim and fluazifop (Group 1)
• ALS inhibitors like rimsulfuron (Group 2)
Many growers I’ve spoken to utilize glyphosate and grass-selective herbicides to control johnsongrass in their orchards, but ALS inhibitor use specifically on johnsongrass in orchards and vineyards is not common. Research trials I installed this summer indicate all three of these herbicides can be similarly effective for post-emergent applications, though rimsulfuron was not as consistent as the others, an effect that is likely dependent on the size of the johnsongrass at treatment. Though glyphosate gave the best above-ground kill of the three, by the end of the trial (60 days after treatment) no differences in johnsongrass weed coverage or biomass was identified between treatments.
Though I received inconsistent results from my post-emergent rimsulfuron treatments, ALS inhibitors like rimsulfuron are interesting herbicides because they often have both soil residual activity and post-emergent activity. Soil residual herbicides, also called preemergent herbicides, can act on weeds as they begin to germinate, killing the plants when they are at their most vulnerable stage. In orchards and vineyards, rimsulfuron herbicides (e.g., Matrix, Revolt, Hinge) are often used in combination with other soil residual herbicides like indaziflam (Alion), flumioxazin (Chateau) and oxyfluorfen (Goal) during the winter months, allowing long-term control of annual weeds far into the growing season. In other crops like processing tomatoes, rimsulfuron herbicides are applied for their post-emergent activity and realize little of their residual potential due to the absence of rainfall to activate the herbicide in the soil. Rimsulfuron herbicides are commonly labeled for use to control johnsongrass seedlings, but my recent research results have demonstrated that these herbicides also have suppressive effects on established johnsongrass populations when applied at particular timings.

What Preemergent Activity Does Rimsulfuron Have on Established Johnsongrass?
To provide better recommendations on how to best use rimsulfuron and other residual herbicides to target johnsongrass, I established a set of trials in prune and walnut orchards in Shasta and Tehama counties in winter and spring 2025. I had two primary goals:
• Test if early spring rimsulfuron applications were effective for control of johnsongrass.
• Determine whether treatments were primarily affecting seedlings or established populations.
In winter (early February), I applied preemergent herbicides, including either fluridone (Brake On!, 43 fluid ounces per acre) or oxyfluorfen (Goal 2XL, 5 pints per acre) as well as an untreated control, to account for any seedlings that might germinate through the duration of my trials. In spring (early March), I applied either rimsulfuron (Revolt, 2 or 4 ounces per acre) or glyphosate (Roundup Powermax 3, 2 pt/A) just before or after shoot emergence from underground rhizomes. Glyphosate was used as a control treatment to account for any post-emergent activity of rimsulfuron on emerged shoots to evaluate only the soil residual effects of rimsulfuron. Very little plant material was available for direct absorption of herbicide through leaf tissue at the spring timing, so this is not a preferred use of glyphosate but was simply used as a more suitable control than untreated johnsongrass. Glyphosate can be very effective in the spring but requires adequate leaf material for absorption into the plant. All together four trials were installed, at three different locations. All treatments were accompanied by a half inch of rain within a week of application, washing the herbicide off thatch and leaves and down into the soil.

In all cases, the rimsulfuron treatments were responsible for most of the weed control in these plots. Plots that received winter preemergent treatments were only marginally different from plots that were untreated in the winter, if followed by glyphosate in the spring. Only plots that received rimsulfuron in spring had any significant reduction in johnsongrass coverage or biomass. This suggests the effects of rimsulfuron observed on johnsongrass populations were not primarily from soil residual effects on seedlings but that the perennial rhizomes themselves were affected. Winter treatments added additional johnsongrass control when combined with rimsulfuron only in two of the four trials, and this effect was seen later in the spring as seedlings began to germinate.
The primary takeaway from this research was that rimsulfuron applications in early March resulted in about a 50% reduction in johnsongrass weed coverage and biomass that lasted past the end of the study, which was in early May. This was a reduction relative to the control treatment with glyphosate alone, suggesting post-emergent absorption of rimsulfuron is likely not the primary mechanism of control but rather activity in the soil. Rates of 4 ounces per acre were much more effective than 2 ounces per acre, and the addition of glyphosate to the tank mix seemed to slightly increase efficacy.
These results are not a silver bullet for johnsongrass management but can fit in with an integrated management plan. Glyphosate and grass-selective herbicides provide consistent mid-season control and should be used to keep established johnsongrass from setting seed. Burndown herbicides like glufosinate (Rely) or mechanical control can also be utilized to prevent seed setting. Rimsulfuron herbicides seem most effective in early spring just before or after shoot emergence followed by adequate rain or irrigation. It is likely that the window of opportunity for this type of application will persist for much of the spring, but this will take further research to determine. With these considerations, it still takes a good deal of diligence to eliminate a well-entrenched johnsongrass infestation. If you have concerns about herbicide resistance, be sure to reach out to your local UCCE farm advisor.