In organic crop production, fertilizers need to be mineralized by soil organisms before nitrogen becomes plant-available. Many factors can impact this mineralization process, including fertilizer chemical composition, physical fertilizer properties, soil properties and application method1–3. Moreover, microbial activity, including N mineralization, is affected by temperature4,5. Typically, an increase in N mineralization with increasing temperature is expected, but data is lacking to inform management decisions. Anecdotal evidence suggests organic fertilizer products perform differently based on the season in which the product is applied. This begs the question: Do organic fertilizer products show different sensitivities to soil temperature? We put this question to the test in a laboratory incubation study.
Laboratory Incubation Study
We set up a laboratory incubation experiment in which soil was incubated with 12 organic fertilizers at 41, 50, 59, 69 and 77 degrees F for five weeks. Soil was collected from an organic vegetable field near Bakersfield, Calif. The soil was mapped at Milagro (coarse-loamy, mixed, superactive, nonacid, thermic Typic Torrifluvents), with soil analyses showing 64% sand, 18% clay and 0.6% soil organic carbon. The soil was sieved, homogenized and air-dried prior to incubation. Fertilizers were obtained from an organic vegetable grower and representative of commercially available products in the region. Fertilizers were categorized as dairy manure compost, chicken manure compost, bone meal dominated pellets, poultry litter dominated pellets and mixed feedstock pellets. The experiment was set up as a full factorial design, including 5 temperatures and 13 fertility treatments (12 fertilizer products and an unamended control), each replicated 4 times, for a total of 260 experimental units. For each experimental unit, we placed 250g of air-dried soil in plastic containers, rewetted to 60% water holding capacity and pre-incubated at the treatment temperature for seven days. Subsequently, fertilizers were added at a rate of 50 mg N/kg soil, roughly equivalent to incorporating 100 lbs N/acre in the top 6 inches of soil. We collected 5 g of soil from each experimental unit on days 0, 7, 14, 21 and 35 and analyzed for ammonium and nitrate concentration. The percentage of N mineralized was calculated as the difference in N concentration in the treatments receiving fertilizer compared to an unamended control divided by the total N applied.
Key Findings
Lowest N mineralization for dairy manure compost, highest for chicken litter compost
Nitrogen release varied drastically across fertilizer products and temperatures (Figure 1). Most products showed a net release of N, with up to 85% of fertilizer N released within the five-week incubation period. In some cases, we observed net negative mineralization rates up to -59%, indicating fertilizer addition caused immobilization of mineral N from sources other than the fertilizer. Dairy manure compost exhibited notably low N mineralization rates, ranging from -29% to 22%, with a mean of -1.4%. Other studies have found similarly low rates, with values mostly below 10%6–10. The carbon to nitrogen (C:N) ratios of dairy manure composts in our study were 11 and 13, within the range of ratios between 8 and 16 reported for other studies6,8. Mineralization rates for dairy manure compost trend low compared to rates expected based on C:N ratios2. In contrast, chicken manure compost showed higher mineralization rates (between 15% and 77%) with an average of 41%. Mineralization rates of chicken manure composts vary across studies. Rates of 25% and 54%11, 28% and 35%2, 100%12 and 0.4% to 6%13 have been reported. Variation in chicken manure compost mineralization may stem from differences in feedstock quality, including bulking agent and particle size as well as composting practices (e.g., turning and watering)14,15. Our findings indicate chicken manure compost performed similarly or slightly better than poultry litter pellets, though other studies reported higher rates for pellets2,6. High variability between studies underscores the need for case-specific evaluations of fertilizer effectiveness in agriculture.
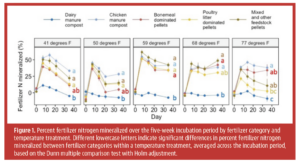
The initial flush of soil organic N mineralization increased with temperature, but not fertilizer N release
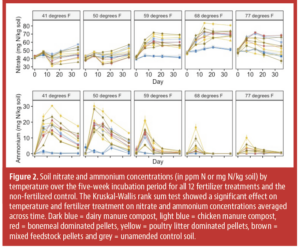
The study found increasing temperature did not significantly increase the percent of N mineralized from fertilizers (Figure 1). In contrast, previous studies show varying responses. For instance, temperature moderately influenced mineralization in one study, increasing N mineralization from 56% to 66% as temperatures rose from 50 degrees F to 77 degrees F16. Another study observed a strong temperature effect on dairy manure N mineralization, with 24% occurring in summer (77 degrees F to 86 degrees F) and only 2% in winter (50 degrees F)10. The difference might be linked to the much higher application rates in those studies compared to this one. Further research is needed on the impact of application rates on N mineralization, especially considering the relevance for optimizing fertilizer timing (single vs split application) and placement (e.g., banding leads to more concentrated fertilizer placement than broadcasting). Like fertilizer mineralization, soil organic N mineralization in our study did not increase with increasing temperature during the 35-day incubation period, though soil mineral N content at the end of the pre-incubation did increase linearly from 42 mg N/kg at 41 degrees F to 49 mg N/kg at 77 degrees F (Figure 2). Other research has shown exponential increases in soil organic N mineralization with temperature beyond the pre-incubation period 5,17. Compared to other studies, our soil had a high sand content (>60%) and very low soil organic carbon content (ca. 0.6%), suggesting the characteristics of the soil and environmental factors like rewetting may play a role in temperature sensitivity of mineralization, which has implications for irrigation management.
Relative performance of fertilizer products varied by temperature
The ranking of mineralization rates among fertilizers varied by temperature (Figure 1). Bonemeal-dominated pellets ranked among the best products in terms of N release at temperatures of 59 degrees F and above, while chicken litter-dominated pellets ranked relatively high at 50 degrees F and 59 degrees F but performed worse above 68 degrees F (Figure 1). Other studies have found differences in the relative performance of organic fertilizers at different temperatures16,18, but clear insights on the fertilizer properties that lead to better performance at low vs high temperature are missing. Studies on decomposition of plant litter suggest decomposition slows down more severely with decreasing temperatures for ‘low-quality’ litter, such as roots, than ‘higher quality’ litter, such as shoots, but findings vary across studies4,19. With respect to N availability of organic fertilizers, not only temperature effects on N mineralization, but also temperature effects on loss pathways need to be considered. Ammonia volatilization is known to increase with increasing temperature and can constitute a substantial loss of N from organic fertilizers, especially in sandy and alkaline soils, but subsurface application and management of soil pH can help reduce loss20,21. Likewise, gaseous N loss through denitrification may be enhanced at higher temperatures22. Thus, organic fertilizer properties can affect both the temperature sensitivity of fertilizer N mineralization and loss pathways for released N. Growers may benefit from fine-tuning organic fertility management by season.
Low temperatures limit nitrification rates
Across fertilizer treatments, nitrate concentrations increased and ammonium concentrations decreased with increasing temperature (Figure 2), indicating an inhibitory effect of low temperature on nitrification as documented by previous studies16,23,24. Soil tests may underestimate plant-available N from organic fertilizers at colder temperatures since they typically report only nitrate. In our experiment, ammonium concentrations reached up to 35 ppm at lower temperatures, equivalent to approximately 70 lbs N/acre in the top 6 inches of soil. While both ammonium and nitrate are plant-available forms of N, the preferred ratio of ammonium to nitrate differs between crops and is affected by soil properties like pH25. Therefore, impacts of reduced nitrification due to low temperature on yield are expected to be context-dependent. In general, ammonium is much less mobile in the soil compared to nitrate26. This suggests placement of organic fertilizer closer to the plant roots (e.g., through banding) may be beneficial to increase N availability during colder periods as long as application rates are low enough to keep ammonium concentrations below toxicity levels27.
Implications for Nitrogen Management in Organic Production
We wanted to find out if different organic fertilizers react differently to changes in soil temperature. Our research showed bonemeal-dominated pellets work better in warmer conditions, while poultry litter-dominated pellets perform better at moderate temperatures. Other studies also highlight temperature and soil type can impact how well fertilizers work. In general, our results caution against assuming fertilizers release N faster at higher temperatures. Mineralization of dairy manure compost was very low, even when considering the relatively high C:N ratio of 11 to 13, making it more useful for improving soil quality rather than as a primary fertilizer. Lower temperatures inhibited nitrification, which has been well documented in other studies. Accumulation of mineralized N in the ammonium pool at lower temperatures has important implications for soil testing and strategic fertilizer placement. Implications for yield are expected to be crop- and soil-dependent. Future research directions include how soil types, the impact of temperatures above 77 degrees F and higher rates of fertilizer affect N mineralization rates.
References
1. Cassity‐Duffey, K., et. al. Soil Science Society of America Journal 84, 534–542 (2020).
2. Lazicki, P., Geisseler, D. & Lloyd, M. Journal of Environmental Quality 49, 483–495 (2020).
3. Smith, R., et. al. California Agriculture 76, 77–84 (2022).
4. Fierer, N., et. al. Ecology 86, 320–326 (2005).
5. Miller, K., et al. Communications in Soil Science and Plant Analysis 50, 77–92 (2019).
6. Hartz, T. K., Mitchell, J. P. & Giannini, C., HortScience 35, 209–212 (2000).
7. Hurisso, T. T., et al. Organic Agriculture 2, 219–232 (2012).
8. Hurisso, T. T., et al. Agrosystems, Geosciences & Environment 7,
e20555 (2024).
9. Lehrsch, G. A., et al. Nutrient Cycling in Agroecosystems 104, 125–142 (2016).
10. Watts, D. B., Torbert, H. A. & Prior, S. A. Soil Science 175, 27 (2010).
11. Geisseler, D., et. al. Journal of Environmental Quality 50,
1325–1338 (2021).
12. Johnson, H. J., et al. HortTechnology 22, 37-43 (2012).
13. Tyson, S. C. & Cabrera, M. L. Communications in Soil Science and Plant Analysis 24, 2361–2374 (1993).
14. Leconte, M. C., et al. Biology and Fertility of Soils 47, 897–906 (2011).
15. Shi, W., et al. Applied Soil Ecology 11, 17–28 (1999).
16. Hartz, T. K. & Johnstone, P. R. HortTechnology 16, 39-42 (2006)
17. Dessureault-Rompré, J. et al. Geoderma 157, 97–108 (2010).
18. Thangarajan, R., et al. Environmental Science and Pollution Research 22, 8843–8854 (2015).
19. Conant, R.T., et al. Global Change Biology 17, 3392–3404 (2011).
20. Terman, L. Advances in Agronomy 31, 189–220 (1979).
21. Wester-Larsen, L., et al., Journal of Environmental Management 323, 116249 (2022).
22. Dai, Z. et al. Global Change Biology 26, 5267–5276 (2020).
23. Sims, J. T. Journal of Environmental Quality 15, 59–63 (1986).
24. Tisdale, S. L. Soil Fertility and Fertilizers (1993).
25. Chen, J. et al. Field Crops Research 307, 109240 (2024).
26. Norton, J. & Ouyang, Y. Front Microbiol 10, 1931 (2019).
27. Esteban, R., et al. Plant Science 248, 92–101 (2016).