One fortunate aspect of working in agriculture is that hot summer days are soon followed by harvest season and the onset of nicer weather. Now is the time to start thinking about postharvest soil fertility maintenance. Dry, granular phosphorus and potassium fertilizer products (e.g., monoammonium phosphorus (MAP:11-52-0); sulfate of potash (SOP: 0-0-60); and muriate of potash (MOP: 0-0-50)) are popular choices for postharvest fertility work and a cornerstone of annual nutrient budgets for many crops.
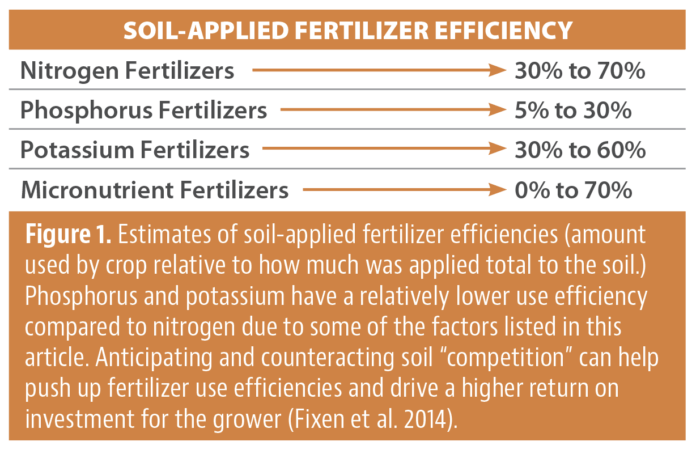
These fertilizer formulations are popular with growers as they can apply bulk nutrients to help maintain soil fertility and get a jump start on supporting the next crop cycle. A goal for growers and their advisor network is to manage the applied nutrients so that much of it ends up in the crop and fertilizer uptake efficiency is optimized. With that in mind, there are various sources of “competition” that prevent the uptake of the nutrient by the plant, which leads to inefficiency in fertilizer use (Fig. 1).
In a perfect world, 100% of your fertilizer application would be used by the crop to produce yield and fertilizer losses would be minimized. However, as Figure 1 shows, use efficiency of fertilizers vary greatly by nutrient category. In this article, we will name the various efficiency loss pathways, or “competition”, for your phosphorus and potassium fertilizer applications so you can work with a crop advisor to try and work around them. Before we jump in, let’s start with some phosphorus and potassium 101.
Why Crops Need Phosphorus and Potassium
Phosphorus and potassium are plant macronutrients that are required in large quantities by the crop to produce a high-quality yield. However, phosphorus and potassium play different roles in the plant. Phosphorus is a crucial component for converting solar energy into food, fiber and other plant products. Furthermore, phosphorus also plays a key role in the metabolism of sugars, energy storage and transfer, cell growth and the transfer of genetic information. An interesting bit about phosphorus is that one can point out the element embedded in the actual chemical structures the plant is using to create yield. For example, you can point to the actual phosphorus that is used to build the biological currency plants use to “pay” for work (ATP) and the phosphorus backbone that all crops use to construct their genes (DNA).
On the other hand, potassium is much more difficult to point out in the crops’ molecular portfolio. However, we do know that potassium is still needed by the plant to support high yields. Briefly, potassium increases crop yield, improves crop quality, reduces disease and decreases risk of lodging and branch breakage, etc. Except for nitrogen, plants often require more potassium than any other nutrient as it plays vital roles in crop growth and development.
Understanding the Competition
With the basics out of the way, we can now turn our attention to the factors that reduce your fertilizer uptake efficiency by the crop. I will refer to the factors as “competition” because these factors interfere with your best laid fertilizer plans and will reduce your crops’ ability to use the nutrient. This will impact the return on your fertilizer investment when harvest comes around.
Phosphorus Competition
Phosphorus can be a tricky nutrient to manage because it interacts with other soil elements that will reduce overall fertilizer use efficiency (Fig. 2). Due to these interactions, the fertilizer use efficiency range can be quite low. For example, only 5% to 30% of the fertilizer might get used by the crop during the growing season (Fig. 1). The rest is lost to the competition.
Slow Diffusion Rates
Diffusion refers to the movement of phosphorus from the bulk soil toward the roots by way of a concentration gradient. Other plant uptake pathways exist but diffusion is the major pathway for phosphorus. For example, research shows that corn acquires ~93% of its phosphorus needs for the season via diffusion (Barber 1995; Fernandez 2016). What’s the catch? The rate of diffusion is sensitive to several factors that reduce the speed of how fast the process, or rate, can occur. These soil factors include cold temperatures, compaction, saturated or dry conditions. When these factors reduce the diffusion rate, they can compete with your fertilizer application by not allowing the crop to be adequately supplied with phosphate.
Mineral Antagonisms
In some cases, the over application of other nutrients can prevent plant uptake of phosphorus, which will reduce fertilizer use efficiency. For example, research shows that excessive applications of calcium and magnesium can interfere with the crop’s ability to use phosphorus (Better Crop 1999).

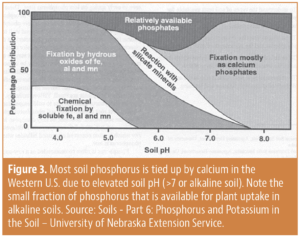
Soil pH
Competition for your phosphorus fertilizer efficiency can be influenced by soil pH and the conditions that result. For example, when soil calcium and phosphorus fertilizer come together under alkaline conditions (pH >7), they form a hard mineral called apatite, which is the same mineral that your bones and teeth are made of. Once this mineral fixation happens, the bound phosphorus is now unavailable for plant uptake, with reduces use efficiency. Figure 3 shows just how much soil phosphorus is made unavailable by the calcium complexes (up to 80% to 90% reduction in availability).
Runoff/Erosion
Phosphorus can bind to soil particles and move off the field during rainfall or during snowmelt. When soil leaves the field (erosion), it is more than likely carrying some phosphorus with it, reducing use efficiency. This impact is more pronounced on sloped fields or in areas where heavy rainfall occurs.
Poor Recommendations
This might not be an obvious source of competition, but how your local ag lab analyzes the pool of soil phosphorus matters. Briefly, soil phosphorus can be measured by three different methods: Bray, Mehlich and Olson. Your local soil pH should determine the extraction method as each works best within a certain pH range. A mismatch in method will overestimate or underestimate the phosphorus supply. The wrong test will lead to a bad fertilizer recommendation, which can be inefficient. In this case, your competition is bad information.
Potassium Competition
Now let’s turn our attention to the competition for potassium. Crops are generally able to use more of the potassium provided by a fertilizer application relative to phosphorus. For example, 30% to 60% of potassium fertilizer might get used by the crop (Fig. 1). The rest is lost to the competition (Fig. 4).

Potassium ‘Tie Up’ by Clays
Soils differ in their relative amounts of sand, silt and clay particles (e.g., texture), and this distribution impacts several important soil properties. For example, clay-dominated soils tend to have a high cation exchange capacity (CEC), which is the relative ability of a soil to store positively charged nutrients. However, nutrient release rates are inversely related to the CEC value. In this sense, high clay soil will release stored potassium back to soil solution at a slower rate relative to sandy soil. Thus, clays can temporarily ‘tie up’ potassium fertilizer applications by storing it and then only slowly releasing it back to the soil for plant use, which can decrease efficiency.
Potassium Fixation
This next competitor occurs under unique circumstances and has a longer lasting impact on your fertilizer program. Certain clay minerals can scavenge potassium from the soil, including your potassium fertilizers, and render the nutrient unavailable for plant use. This process is called potassium fixation. Strong potassium fixation capacity is typically associated with the presence of vermiculite and mica-based materials in soil, in a specific age class, and from soils derived from granite parent material (Pettygrove and Southard 2003). Fixed potassium, once tied up, is no longer available for plant use during the growing season and can have a serious impact on fertilizer use efficiency.
Leaching
Sandy soils are poor at storing nutrients (e.g., low CEC). Furthermore, they also have a high nutrient release rate back to the soil. Under these conditions, potassium fertilizers can leach out of the root zone, which reduces the fertilizer use efficiency. Thus, excessive rainfall or irrigation sets on sandy, low-organic-matter ground can compete with your crop for potassium.
Dry Soils
Unlike phosphorus, ~20% of potassium is moved to the plant through the soil moisture in a process called mass flow (Barber 1995; Fernandez 2016). Excessively dry soils at any depth throughout the rooting zone will prevent potassium from reaching the plant and reduce the fertilizer use efficiency.
Antagonisms with Sodium
This section applies to those struggling with excessive sodium in the irrigation water or soil. In this case, the sodium can compete with your potassium fertilizer plan by interfering with the crops’ ability to acquire the nutrient. Wakeel (2013) recently summarized the antagonistic effect of sodium on potassium uptake in detail. Briefly, excess sodium makes it difficult for potassium to be taken up by the crop and sodium also increases potassium leakage from the crop, which reduces use efficiency.
Outsmarting the Competition
Now that we have named the competition, we can work to manage them to minimize their impact and get more of your applied nutrients into the crop where it belongs. Where to begin? Much of the competition mentioned above can be identified through routine soil testing and from sound advice from a local crop advisor. By using an updated soil report, you and your advisory team can be on the lookout for conditions that might give your fertilizer competition a leg up in the field so you can manage them appropriately. For some other situations, a call to the local lab can confirm they are using the right extraction method and generating the correct fertilizer recommendation. Local geological and soil series reports, if you suspect potassium fixation, can be invaluable for understanding conditions in your field that could be competing for your potassium. As reviewed here, several competitors are working to reduce the use efficiency of your expensive fall fertilizer applications. If you are concerned about improving nutrient uptake in the crop and keeping the phosphorus and potassium out of the hands of the competition, consider contacting a local crop advisor to help develop a field-specific management plan. Once your competition is formally identified, using this article as a guide, it can be better managed on your farm and fertilizer use efficiency for phosphorus and potassium can be increased.
Dr. Karl Wyant serves as the Director of Agronomy at Nutrien. Please visit www.nutrien-ekonomics.com for the latest in nutrient management topics and other free resources.
References
Fixen et al. 2014. Nutrient/Fertilizer Use Efficiency: Measurement, Current Situation and Trends
Better Crops/Vol. 83 (1999, No. 1). Phosphorus Interactions with Other Nutrients
Wakeel 2013. Potassium–sodium interactions in soil and plant under saline-sodic conditions



















