Soil salinization is caused by excessive accumulation of salts in the soil and is one of the most severe land degradation problems. Globally, salt-affected soils are estimated to be about 2 billion acres and are expected to increase in the future. In California, about 4.5 million acres of irrigated cropland (more than half) are affected by salinity, causing significant problems to the state’s agriculture. When salinity levels exceed critical thresholds in the soil, the plants cannot reach their full genetic growth potential, development and reproduction.
Causes and Consequences of Soil Salinity
Soil salinity can be related to the origin of the soil (e.g., in soils from land that was once submerged under the level of the sea or a lake.) It can also be caused by humans. Irrigation in dry areas exacerbates the problem as water contains salts that are left in place after evapotranspiration, and there is not enough rainfall to help with the leaching (i.e., the process of washing off excess salts from the surface toward the deeper layer of the soils and out of the reach of plants.)

Salinity can be caused by excess in different kinds of salts, including table salt (NaCl), potassium chloride (KCl), etc. Table salt is the most common and most problematic. It is composed of sodium ions (Na+), which negatively impact soil physical-chemical properties and create an osmotic stress in plants, and chloride anions (Cl-), which are toxic to plants.
Accumulation of sodium in soils causes swelling of clays, destroys soil structure and reduces infiltration and water holding capacity (Figure 2). This reduces the oxygen & water availability to roots. Excess of salts in the soil generates osmotic stress, which affects grapevine physiology and performance. At low rates, it is a chronic problem that disturbs grapevine water relations, causes stomatal closure in grapevine and reduces leaf and crop size. At high rates, it can create toxicity problems that can lead to premature leaf senescence and plant death (Figure 1, see page 22).
Salt buildup can result in three types of soils: saline, saline-sodic and sodic. Salinity & sodicity terms are used interchangeably very often. However, salinity refers to the concentration of salts (of all kinds) in the soil. Sodicity is associated with the proportion of sodium in pore water or adsorbed to the mineral surface. In saline soils, there are enough soluble salts of all kinds to injure plants. Sodic soils are low in soluble salts but relatively high in exchangeable sodium. Saline-sodic soils have a high content of salts and high content of sodium relative to calcium and magnesium salts.

The electrical conductivity of soil extracts can measure soil salinity, as with more salts in the water, it is easier for the current to flow. Sodicity of soil is indicated by the exchangeable sodium percentage (ESP), which is the soil cation exchange capacity occupied by sodium, or by the measure of sodium adsorption ratio (SAR), which represents the amount of sodium with respect to calcium and magnesium. Saline, sodic and saline-sodic soils can be differentiated according to their physical-chemical properties. Soils with EC > 4 dS/m, SAR <13, pH <8.5 are classified as saline soils; soils with EC <4 dS/m, SAR >13, pH >8.5 are classified as sodic soils; and soils with EC >4 dS/m, SAR >13, pH <8.5 are classified as saline-sodic soils.
Alleviating Soil Salinity
Alleviation of salt-related problems is of crucial importance to reduce the impact on crop performance and ensure the profitability of agriculture. This can be done by decreasing the amount of Na+ ions, destroying soil structure and replacing them with Ca+ ions. Adding calcium helps maintain a favorable electrolyte concentration in the soil solution, thereby preserving the physical and chemical properties such as structural stability and clay flocculation, which encourages better root penetration and air and water movement through the soil. Ca2+ ions have a much higher flocculating power than sodium and potassium ions due to their charge and size. Thus, Ca2+ ions have a higher affinity for clays and can easily replace Na+ ions during soil reclamation practices to improve soil properties.
For this purpose of alleviating salt buildups, soils are treated with calcium-based amendments, the most common being the application of gypsum (CaSO4·2H2O). Other products, such as calcium chloride (CaCl2), calcium carbonate (CaCO3) and sulphuric acid (H2SO4), can also be used for reclamation of saline soils but are not as effective as gypsum, and they are destined to specific conditions.
Calcium chloride contains chloride anions that are known to be toxic to plants and, in particular, grapevines. Sulphuric acid does not directly contain calcium required to replace the Na+ ions in the soil. Hence, it is only effective in calcareous soils (rare in the U.S.), where it can react with the calcium carbonate to free the calcium already in place. Calcium carbonate (CaCO3), or lime, is alkaline and significantly less soluble than gypsum. It is generally used to reclaim acid soils. It cannot provide sufficient Ca2+ ions for effective Ca2+- Na+ exchange as it can free Ca2+ ions only in acid soils, while salt-affected soils are generally alkaline.
Gypsum as a Solution
Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate with the chemical formula CaSO4∙2H2O containing two molecules of water inside every single crystal.
Calcium sulfate can also be found in two more phases: calcium sulfate anhydrite (CaSO4), which does not contain water of crystallization, and calcium sulfate hemihydrate, also called bassanite (CaSO4∙1/2H2O), which only contains a half-molecule of water per crystal. Severe pressure, temperature and other natural conditions make gypsum outcrops lose water molecules and form anhydrite and bassanite.
Besides a high concentration of calcium and sulfur, pure-quality gypsum contains 21% water, giving it peculiar properties (e.g., different solubility) with respect to the other forms of calcium sulfate. Of particular importance under dry conditions is the ability of plants to access and use the water in the crystalline structure of gypsum.
The use of gypsum crystallization water by organisms is a critical water source for life under dry conditions, as recently demonstrated by Palacio et al. 2014. These authors reported that in natural conditions and during summer, the sap of shallow-rooted plants is 70% to 90% derived from gypsum crystallization water. The extraction and use of water molecules from gypsum by plants is accelerated in hot temperatures. Microporosity of gypsum also offers protection to the soil microbes and promotes relatively abundant and diverse microbial life in dry conditions. Soil microbes may in turn increase the availability of inorganic compounds to plants.
Gypsum is best applied routinely, as frequent irrigations leach out calcium from the root zone. This is even more important when water for irrigation is alkaline. If gypsum is not applied regularly and calcium content decreases in the soil, the soil tends again to get compacted and the infiltration of water slows down, creating stress to plants.
It is recommended to spread gypsum on soils as an application through low-volume irrigation sources (i.e., drip and sprinklers) and is shown to work best with irrigation water of low salinity, i.e., about 0.1 ds/cm. Liquid gypsum application can increase the water infiltration to greater depths under the emitters over time due to soil particle binding and aggregation properties of calcium. When gypsum is applied through drip lines with irrigation waters of high bicarbonate content, it requires cautionary measures and lowering of pH (<6.5) because of the formation of calcium bicarbonate precipitates.
Gypsum Research
With the intent of clarifying the effectiveness of gypsum to reclaim salt-affected soils and to provide application guidelines in vineyards, we performed a three-year-long project where we monitored the response of soil physics, grapevine physiology and fruit composition to different dosages and forms of CaSO4 (anhydrite, CaSO4 & gypsum CaSO4∙2H2O) in synergy with organic matter.
The experiment was performed from 2019 to 2021 in a Merlot vineyard located in a fine loamy sodic soil on the southwest side of Bakersfield, Calif. After the first season of measurements (2019), to ensure no differences across treatments before application, we broadcasted chemical amendments in winter 2020 in bands under the vines (Figure 3, see page 26). The experiment was a completely randomized block design with six treatments replicated four times, and each replicate was 0.2 acres large.
The treatments were applications of gypsum at different rates: 2.5 t/ac (50% reclamation rate, code 50G), 5.1 t/ac (100% reclamation rate, code 100G), 10.2 t/ac (200% reclamation rate code 200G), application of anhydrite at 5.1 t/ac (100% reclamation rate, code 100A) and addition of compost to gypsum (5.1 t/ac gypsum + 1t/ac compost (code 100GC)). We included one untreated control (code CTRL). All treatments were applied in one single application at the beginning of the experiment.
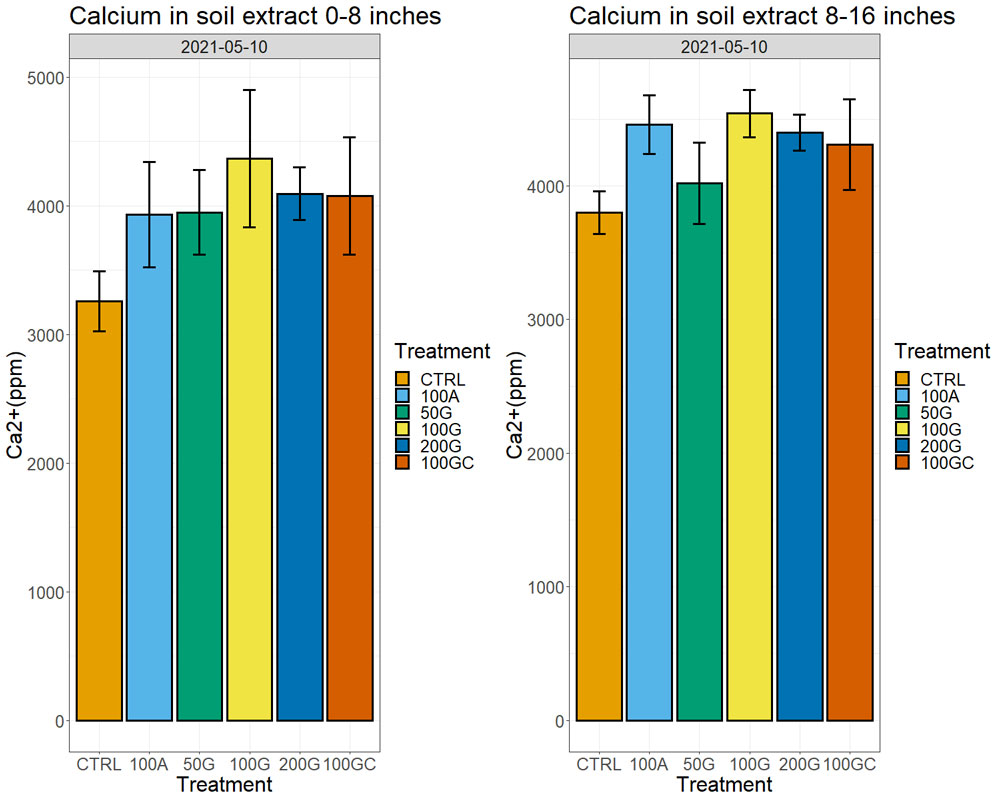
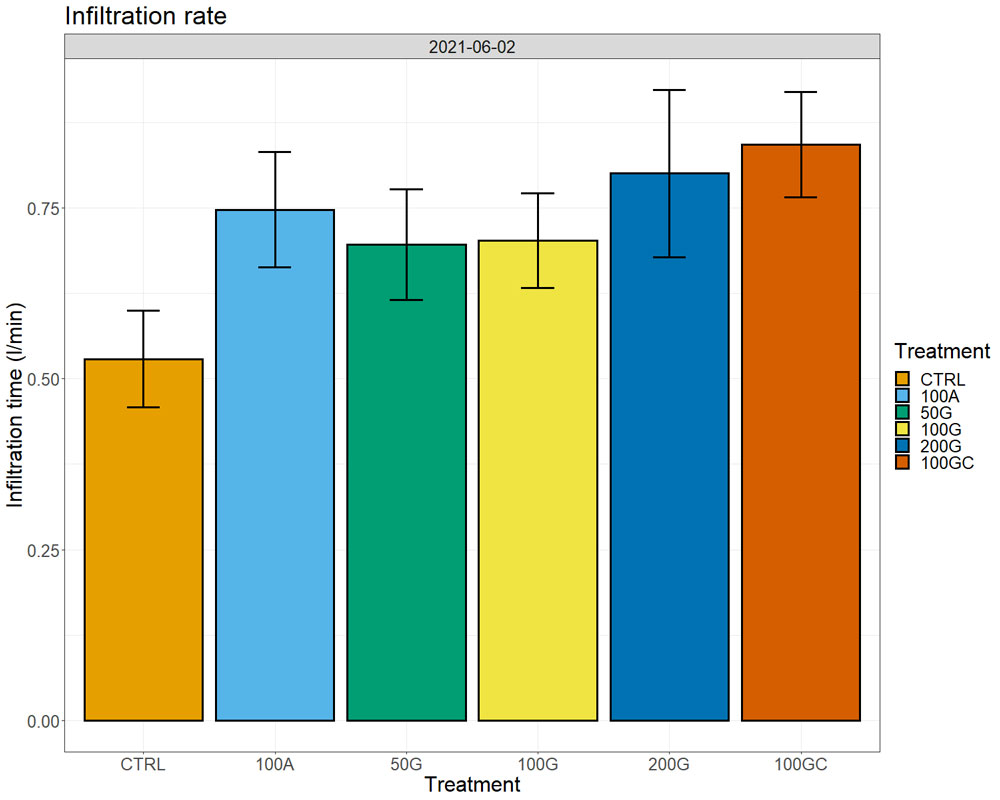
Calcium amounts in the soil extracts (obtained after treatment of soil samples with ammonium acetate) increased in all treatments with respect to the control one year after the application, both in the top eight inches and at a depth between 8 and 16 inches. We observed the largest increase in the 100G, which had 28% more exchangeable calcium ions on the soil particles than the control in the top eight inches and 15% more in the 8- to 16-inch depth (Figure 4, see page 26). Sixteen months after the application, we started observing effects on water infiltration rate in the soil, with the untreated control showing the lowest water infiltration rate. The 200G and 100GC showed the highest infiltration rate with 65% and 63% improvement over the control, respectively (Figure 5, see page 26).
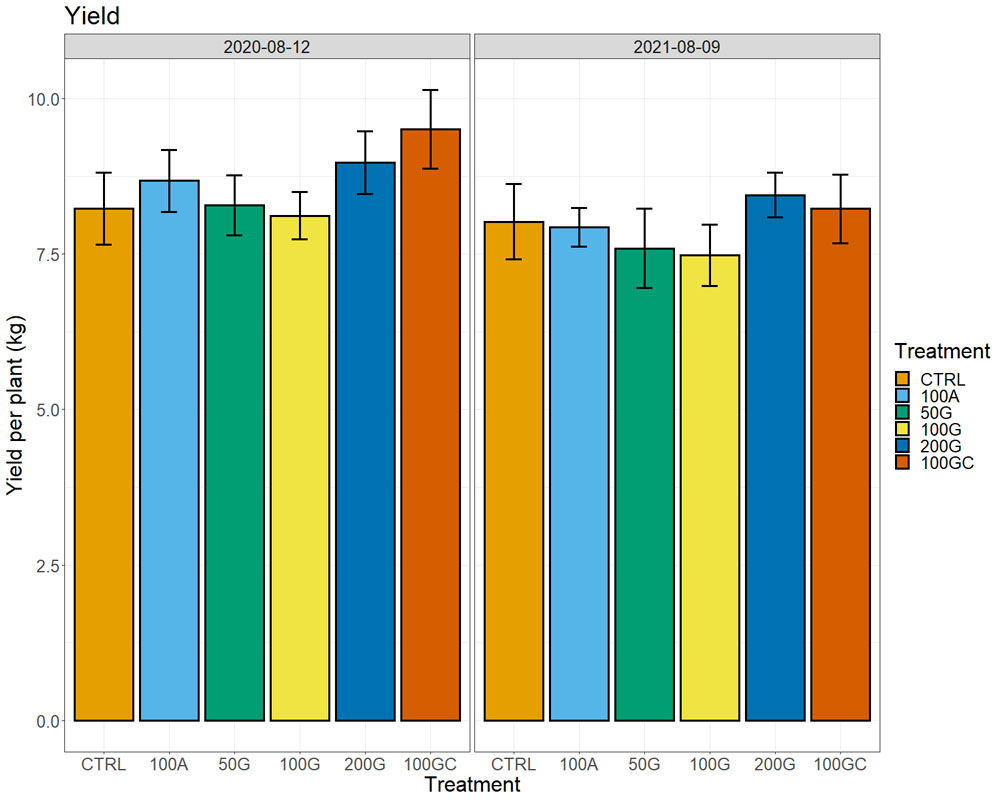
We did not observe notable differences in plant water status measured by a pressure chamber (water potentials), neither in photosynthesis nor transpiration across the treatments. In the two years after the treatment, we observed higher yields in the 200G and 100GC with respect to all other treatments and the control (Figure 6). The response was more robust in the year of the application, in particular for the 100GC, with plants producing 15% higher yield than the control, but only 2% more than the control in the second year. At the same time, the effect of the 200G was more consistent and was higher (8%) with respect to the control the same year as well as the following year (5%). The other treatments did not have positive effects on yield. We did not observe significant differences in sugar content (Brix), pH or titratable acidity of musts across treatments.
This trial showed that gypsum effectively reduces the adverse effects of salt stress on soils and vines by increasing calcium ions at the surface of clays. The increase of calcium ions improves soil structure with positive returns on infiltration rates and the increase in soil health promotes grapevine performance and increases yield. The addition of compost enhances the positive effects of gypsum.