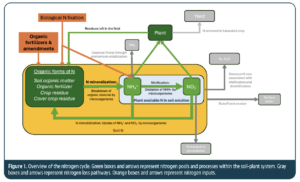
Crop yields are often lower in organic production systems compared to conventional farming systems (De Ponti et al. 2012). This yield difference is partly attributed to challenges in providing plants with the right nutrients, especially nitrogen (De Ponti et al. 2012; Gaskell and Smith 2007). Organic fertilizers must be broken down by soil organisms before N becomes available to plants in the form of ammonium (NH4+) and nitrate (NO3–), a process referred to as N mineralization (Figure 1). The speed and efficiency of this breakdown depend on the properties of the organic fertilizer (Cassity‐Duffey, Cabrera, Gaskin, et al. 2020; Geisseler et al. 2021). For instance, products with lower C:N ratios and liquid products are known to have a faster N release compared to products with higher C:N ratios and dry products, including pellets and composts (Lashermes et al. 2010; Lazicki et al. 2020). With this knowledge, fertilizer products can be chosen to best meet the crop’s N demand. Despite an increasing understanding of N mineralization rates of organic fertilizer, there is still large uncertainty around predicted release rates. One source of this uncertainty surrounds the impact of soil properties. Organic vegetable growers observe variations in nutrient program performance across fields, highlighting the need to understand how soil properties affect organic fertilizer breakdown rates. Research has shown differences in N mineralization from organic fertilizers between soils, but the reasons for these differences are not well understood (Lazicki et al. 2020; Marzi et al. 2020). Soil texture and pH are thought to impact organic fertilizer breakdown (Cassity‐Duffey, Cabrera, Franklin, et al. 2020; Thangarajan et al. 2015), but a thorough assessment of soil properties’ influence is lacking.
Soil Laboratory Experiment
To address knowledge gaps on how soil properties affect N breakdown of organic fertilizers, we determined N mineralization rates of a commonly used 8-5-1 pelletized organic fertilizer in 72 soils with diverse properties (Figure 2). Composite soil samples were gathered from organic vegetable production fields in California’s Santa Maria Valley in fall 2020. This region, located at 34.9530 degrees N, 120.4357 degrees W, is a significant area for vegetable cultivation, boasting a variety of soil types and a Mediterranean coastal climate. Typically, these fields yield two to three crops annually. The valley features several soil series (Table 1), each with its own distinct soil properties.

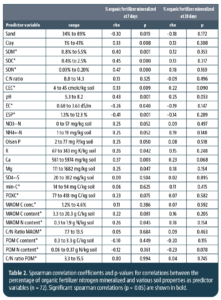
We assessed 25 soil properties (Table 2), including soil texture, soil organic matter, soil organic carbon (SOC), soil organic nitrogen (SON), concentrations of ammonium (NH4+) and nitrate (NO3–) nitrogen, pH levels, electrical conductivity (EC), cation exchange capacity (CEC), exchangeable sodium percentage (ESP), concentrations of phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and sulfate (SO4-), mineralizable carbon (min-C), permanganate oxidizable carbon (POXC), carbon and nitrogen in particulate organic matter (POM) and mineral-associated organic matter (MAOM) fractions. All analyses were conducted using standard methods (Miller et al., 2013; Hurisso et al. 2016; Cotrufo et al. 2019). Mineralizable carbon (min-C), also referred to as soil respiration, is an indicator of microbial activity, while permanganate oxidizable carbon (POXC), also referred to as active carbon, can be considered a microbial food source. Both min-C and POXC are commonly used as indicators of soil health (Moebius-Clune et al. 2016; Norris et al. 2020). Meanwhile, POM and MAOM fractions can be interpreted as labile and more stabilized soil organic matter pools, respectively (Cotrufo et al. 2019; Lavallee et al. 2020).
All 72 soil samples were incubated for 28 days at 25 degrees C and 60% water holding capacity, both with and without the addition of organic fertilizer. The organic fertilizer used was a pelleted mix derived from meat and bone meal, poultry manure and feather meal (8-5-1), applied at a rate of 50 mg N per kg soil. This application rate corresponds to approximately 100 kg N per hectare (89 lbs. per acre), assuming a soil density of 2,000,000 kg per hectare in the top 15 cm of soil, consistent with rates reported by growers using split applications. Before application, the fertilizer was ground into a fine powder and thoroughly mixed. We measured NH4+ and NO3– concentrations on days 0, 7 and 28 of the incubation. The percentage of fertilizer N mineralized after 7 and 28 days was calculated as the difference in mineral N (NH4+-N + NO3–-N) over time between samples with and without fertilizer, divided by the amount of fertilizer N applied (Lazicki et al. 2020).
Key Findings and Implications
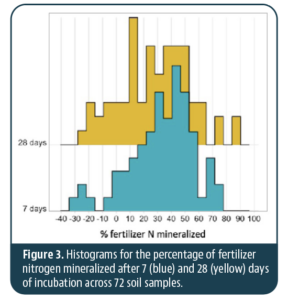
We observed a wide variation in the percentage of N mineralized after 7 or 28 days of incubation (Figure 3). The mineralization rates ranged from -31% to 78% after 7 days and -22% to 87% after 28 days. Negative values indicate N was tied up in the soil after fertilizer application. On average, across all soils, 32% of the fertilizer N was mineralized after 7 days and 27% after 28 days. These results suggest soil characteristics can have a large impact on N mineralization from organic fertilizers.
Our findings are consistent with previous research. For example, a study comparing 22 commercial organic fertilizers found N mineralization ranged from 25% to 93%, with most N being mineralized within the first week. Similarly, in our study, the largest portion of fertilizer N was released within the first 7 days, with more subtle changes between days 7 and 28. Negative rates of N mineralization from organic amendments are found for inputs with high C:N ratios, but other studies typically find net mineralization for commercially available organic fertilizers. It’s important to note the application rate of organic fertilizer in our study was low compared to many other studies, so it is possible the balance between mineralization (release) and immobilization (tie-up) of N may depend on the application rate.
We found several significant correlations between soil properties and the percentage of fertilizer N released after seven days of incubation. For example, N mineralization after seven days was positively correlated with clay content, SOM, SOC, SON, CEC, pH, nitrate nitrogen, Olsen phosphorus, soil potassium, calcium, magnesium and microbial biomass nitrogen content. This suggests that fostering soil organic matter or soil carbon and general soil fertility may benefit the efficiency at which organic fertilizers are mineralized, especially during the first week following fertilizer application. In contrast to our expectation, the soil health indicators min-C and POXC and the organic matter fractions POM and MAOM performed poorly in predicting organic fertilizer mineralization in our study. Significant negative correlations were found with sand content, electrical conductivity and exchangeable sodium percentage. Negative effects of salinity and sodicity on organic fertilizer mineralization imply a compounding challenge for growing crops in saline or sodic soils under organic management.
Our study demonstrates organic fertilizer mineralization is influenced by soil properties. Maintaining pH, EC and ESP within the optimum range is important to ensure sufficient N mineralization from organic fertilizer. Fertilizer mineralization rates were greater at higher soil organic matter and soil nutrient concentrations, highlighting the importance of building these for efficient fertilizer use in organic crop production. Future research should focus on management practices that are most effective and cost-efficient for building soil organic matter and fertility to support reduced organic fertilizer input costs in the medium and long term. Our findings suggest in organic production systems, nutrient management should consider not only plant nutrient demand but also the nutrient demand for building and maintaining soil health and fertility.
The authors are grateful for support from Betteravia Farms for soil sample collection and analysis. Songyi Kim provided support through the Korea National Institute for International Education, Ministry of Education. Finally, this project could not have been completed without the technical support of Craig Stubler for laboratory analyses.
References
Al-Busaidi, K. T., Buerkert, A., & Joergensen, R. G. (2014). Carbon and nitrogen mineralization at different salinity levels in Omani low organic matter soils. Journal of Arid Environments, 100, 106-110.
Bowles, T. M., Hollander, A. D., Steenwerth, K., & Jackson, L. E. (2015). Tightly-coupled plant-soil nitrogen cycling: comparison of organic farms across an agricultural landscape. PloS one, 10(6), e0131888.
Cassity‐Duffey, K., Cabrera, M., Franklin, D., Gaskin, J., & Kissel, D. (2020). Effect of soil texture on nitrogen mineralization from organic fertilizers in four common southeastern soils. Soil Science Society of America Journal, 84(2), 534-542.
Cassity‐Duffey, K., Cabrera, M., Gaskin, J., Franklin, D., Kissel, D., & Saha, U. (2020). Nitrogen mineralization from organic materials and fertilizers: Predicting N release. Soil Science Society of America Journal, 84(2), 522-533.
Cotrufo, M. F., Ranalli, M. G., Haddix, M. L., Six, J., & Lugato, E. (2019). Soil carbon storage informed by particulate and mineral-associated organic matter. Nature Geoscience, 12(12), 989-994.
Daly, A. B., Jilling, A., Bowles, T. M., Buchkowski, R. W., Frey, S. D., Kallenbach, C. M., Keiluweit, M., Mooshammer, M., Schimel, J. P., & Grandy, A. S. (2021). A holistic framework integrating plant-microbe-mineral regulation of soil bioavailable nitrogen. Biogeochemistry, 154(2), 211-229.
De Ponti, T., Rijk, B., & Van Ittersum, M. K. (2012). The crop yield gap between organic and conventional agriculture. Agricultural systems, 108, 1-9.
Drinkwater, L. E., & Snapp, S. (2007). Nutrients in agroecosystems: rethinking the management paradigm. Advances in Agronomy, 92, 163-186.
Gaskell, M., & Smith, R. (2007). Nitrogen sources for organic vegetable crops. HortTechnology, 17(4), 431-441.
Geisseler, D., Smith, R., Cahn, M., & Muramoto, J. (2021). Nitrogen mineralization from organic fertilizers and composts: Literature survey and model fitting. Journal of Environmental Quality, 50(6), 1325-1338.
Hartz, T., & Johnstone, P. (2006). Nitrogen availability from high-nitrogen-containing organic fertilizers. HortTechnology, 16(1), 39-42.
Hurisso, T. T., Culman, S. W., Horwath, W. R., Wade, J., Cass, D., Beniston, J. W., Bowles, T. M., Grandy, A. S., Franzluebbers, A. J., Schipanski, M. E., Lucas, S. T., & Ugarte, C. M. (2016, Sep-Oct). Comparison of Permanganate-Oxidizable Carbon and Mineralizable Carbon for Assessment of Organic Matter Stabilization and Mineralization. Soil Science Society of America Journal, 80(5), 1352-1364. https://doi.org/10.2136/sssaj2016.04.0106
Lashermes, G., Nicolardot, B., Parnaudeau, V., Thuriès, L., Chaussod, R., Guillotin, M.-L., Lineres, M., Mary, B., Metzger, L., & Morvan, T. (2010). Typology of exogenous organic matters based on chemical and biochemical composition to predict potential nitrogen mineralization. Bioresource technology, 101(1), 157-164.
Lavallee, J. M., Soong, J. L., & Cotrufo, M. F. (2020). Conceptualizing soil organic matter into particulate and mineral‐associated forms to address global change in the 21st century. Global Change Biology, 26(1), 261-273.
Lazicki, P., Geisseler, D., & Lloyd, M. (2020). Nitrogen mineralization from organic amendments is variable but predictable. Journal of Environmental Quality, 49, 483-495.
Marzi, M., Shahbazi, K., Kharazi, N., & Rezaei, M. (2020). The influence of organic amendment source on carbon and nitrogen mineralization in different soils. Journal of Soil Science and Plant Nutrition, 20(1), 177-191.
Miller, R. O., Gavlak, R., & Horneck, D. (2013). Soil, Plant and Water Reference Methods for the Western Region (WREP-125, Issue.
Norris, C. E., Bean, G. M., Cappellazzi, S. B., Cope, M., Greub, K. L., Liptzin, D., Rieke, E. L., Tracy, P. W., Morgan, C. L., & Honeycutt, C. W. (2020). Introducing the North American project to evaluate soil health measurements. Agronomy Journal, 112(4), 3195-3215.
Pathak, H., & Rao, D. (1998). Carbon and nitrogen mineralization from added organic matter in saline and alkali soils. Soil Biology and Biochemistry, 30(6), 695-702.
Thangarajan, R., Bolan, N. S., Naidu, R., & Surapaneni, A. (2015). Effects of temperature and amendments on nitrogen mineralization in selected Australian soils. Environmental Science and Pollution Research, 22(12), 8843-8854.
Yousif, A., & Abdalla, M. (2009). Variations in nitrogen mineralization from different manures in semi-arid tropics of Sudan with reference to salt-affected soils. Int. J. Agric. Biol, 11, 515-520.




















